
Schistosomiasis
(bilharziasis).
Schistosomiasis is infection with blood flukes of the genus Schistosoma , which are acquired transcutaneously by swimming or wading in contaminated freshwater. The organisms infect the vasculature of the gastrointestinal or genitourinary system. Acute symptoms are dermatitis, followed several weeks later by fever, chills, nausea, abdominal pain, diarrhea, malaise, and myalgia. Chronic symptoms vary with species but include bloody diarrhea (eg, with S. mansoni , S. mekongi , S. intercalatum , and S. japonicum ) or hematuria (eg, with S. haematobium
- Pathophysiology |
- Symptoms and Signs |
- Diagnosis |
- Treatment |
- Prevention |
- Key Points |
- Dermatitis Caused by Avian and Animal Schistosomes |
Flukes are parasitic flatworms that infect various parts of the body (eg, blood vessels, gastrointestinal tract, lungs, liver) depending on the species.
See also the World Health Organization (WHO) and Centers for Disease Control and Prevention (CDC) information on schistosomiasis.
Etiology of Schistosomiasis
Schistosomiasis is by far the most important trematode infection. Schistosoma is the only trematode that invades through the skin; all other trematodes infect only via ingestion. Over 200 million people are infected worldwide (see CDC : About Schistosomiasis).
Five species of schistosomes infect humans; all have similar life cycles involving freshwater snails. S. haematobium causes urinary tract disease; the other Schistosoma species cause intestinal disease.
Geographic distribution of schistosomes that infect humans differs by species:
S. haematobium: Widely distributed over the African continent with smaller foci in the Middle East, Turkey, and India
S. mansoni: Widespread in Africa, foci in Middle East, and the only species in the Western Hemisphere in parts of South America and some Caribbean islands
S. japonicum: Asia, mainly in China, the Philippines, Thailand, and Indonesia
S. mekongi: Southeast Asia
S. intercalatum: Central and West Africa
Humans are the main reservoir of infection. Dogs, cats, rodents, pigs, horses, and goats are reservoirs for S. japonicum , and dogs are reservoirs for S. mekongi . Transmission of these species does not occur within the United States (including Puerto Rico) and Canada, but the disease may be present in travelers and immigrants from endemic areas.
Pathophysiology of Schistosomiasis
Adult Schistosoma worms live and copulate within venules of the mesentery (typically S. mekongi , S. intercalatum , S. japonicum and S. mansoni ) or bladder (typically S. haematobium ). Some eggs penetrate the intestinal or bladder mucosa and are passed in stool or urine; other eggs remain within the host organ or are transported through the portal system to the liver and occasionally to other sites (eg, lungs, central nervous system, spinal cord). Excreted eggs hatch in freshwater, releasing miracidia (first larval stage), which enter snails. After multiplication, thousands of free-swimming, forked-tailed cercariae are released.
Cercariae penetrate human skin within a few minutes after exposure. When they penetrate the skin, they lose their forked tail and transform into schistosomula, which travel through the bloodstream to the liver, where they mature into adults. The adults then migrate to their ultimate home in the intestinal veins or the venous plexus of the genitourinary tract.
Eggs appear in stool or urine 1 to 3 months after cercarial penetration.
Estimates of the adult worm life span range from 3 to 7 years. The females range in size from 7 to 20 mm; males are slightly smaller.
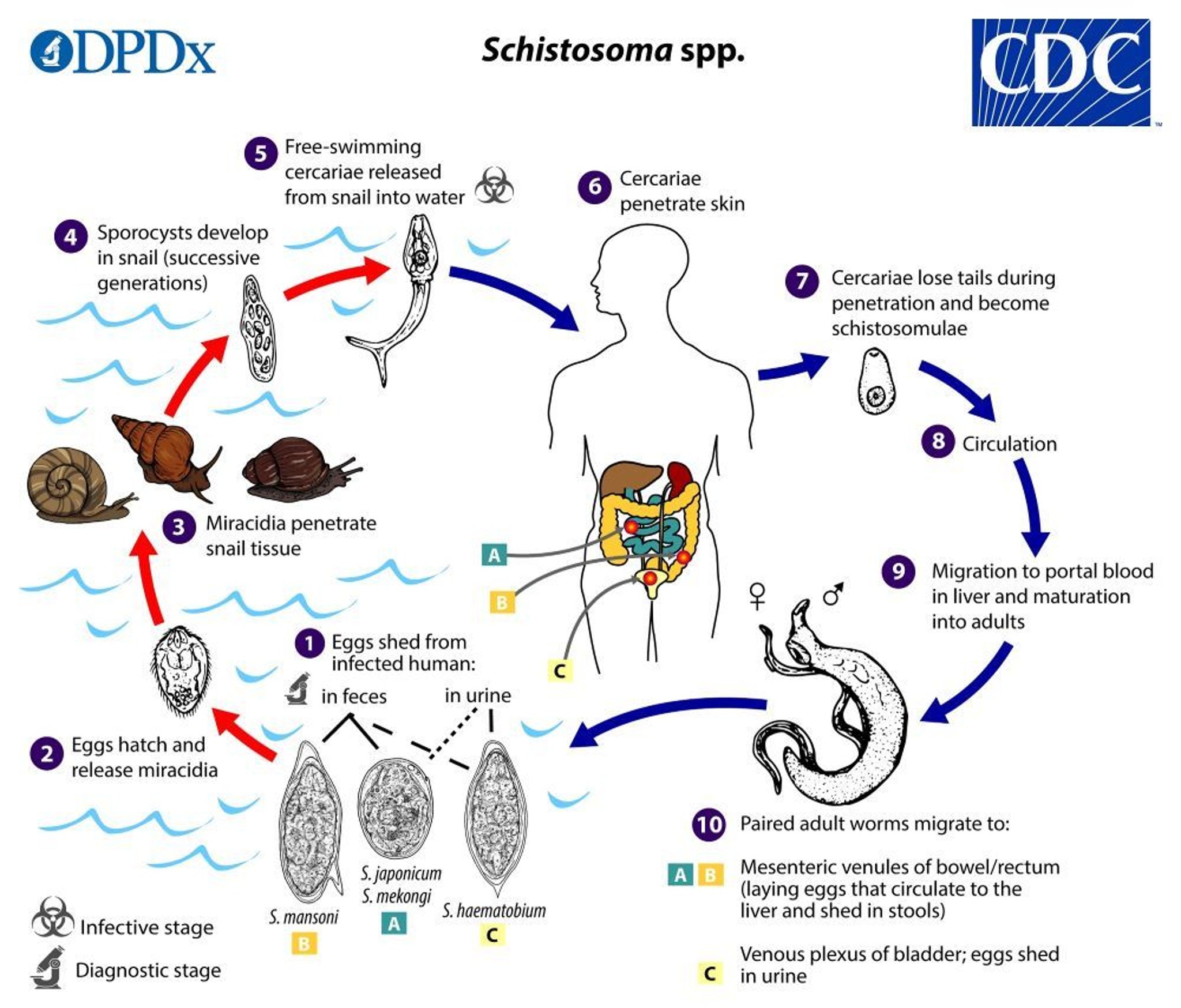
Image from the Centers for Disease Control and Prevention, Global Health, Division of Parasitic Diseases and Malaria.
Symptoms and Signs of Schistosomiasis
Acute schistosome dermatitis.
Most infections are asymptomatic. A pruritic papular rash ( cercarial dermatitis ) can develop where cercariae penetrate the skin.
Acute Katayama fever
Katayama fever is a systemic hypersensitivity reaction that may occur with onset of egg laying, typically 2 to 4 weeks after heavy exposure. Symptoms include fever, chills, cough, nausea, abdominal pain, malaise, myalgia, urticarial rashes, and marked eosinophilia, resembling serum sickness. Manifestations are more common and usually more severe in visitors than in residents of endemic areas and typically last for several weeks.
Chronic schistosomiasis
Chronic infection is most commonly due to repeated exposure in endemic areas but can also occur after brief exposure such as occurs in travelers. Chronic schistosomiasis results primarily from granulomatous host responses to eggs retained in tissues.
Intestinal schistosomiasis: Early on, intestinal mucosal ulcerations caused by S. mansoni , S. japonicum , S. mekongi , or S. intercalatum may bleed and result in bloody diarrhea. As lesions progress, focal fibrosis, strictures, fistulas, and papillomatous growths may develop in the intestine.
Hepatosplenic schistosomiasis: Granulomatous reactions to eggs of S. mansoni , S. japonicum , S. mekongi , and S. intercalatum in the liver usually do not compromise liver function, but they may cause fibrosis and cirrhosis , which can lead to portal hypertension , resulting in splenomegaly , and esophageal varices. Esophageal varices may bleed, causing hematemesis.
Eggs in the lungs may produce granulomas and focal obliterative arteritis, which may ultimately result in pulmonary hypertension and cor pulmonale .
Bladder involvement with S. haematobium produces ulcerations in the bladder wall that may cause dysuria, hematuria, and urinary frequency. Over time, chronic cystitis develops. Strictures may lead to hydroureter and hydronephrosis. Papillomatous masses in the bladder are common, and squamous cell carcinoma of the bladder may develop.
Genital schistosomiasis in young girls and women can involve the vulva, vagina, and cervix as well as the fallopian tubes. Genital schistosomiasis can result in vaginal bleeding during sex and gynecologic examinations, pain during sex, infertility, ectopic pregnancy, abortion, and increased risk of acquiring HIV infection ( 1 ). Male genital involvement of the epididymis, testicles, spermatic cord, or prostate can result in pelvic, coital, or ejaculatory pain, hematospermia, abnormal swelling of genital organs, and infertility.
Blood loss from both gastrointestinal and genitourinary tracts frequently results in iron-deficiency anemia .
Secondary bacterial infection of the genitourinary tract is common with Salmonella , and persistent or recurrent infection may occur. Neurologic complications can occur even in light Schistosoma infections. Eggs or adult worms lodged in the spinal cord can cause transverse myelitis , and those in the brain can produce focal lesions and seizures.
Symptoms and signs reference
1. Orish VN, Morhe EKS, Azanu W, et al : The parasitology of female genital schistosomiasis. Curr Res Parasitol Vector Borne Dis 2:100093, 2022. Published 2022 May 27. doi:10.1016/j.crpvbd.2022.100093
Diagnosis of Schistosomiasis
Microscopic examination of stool or urine ( S. haematobium ) for eggs
Antigen tests
Serologic tests
Diagnostic testing is indicated for patients with symptoms of schistosomiasis and relevant epidemiologic exposure. Screening of asymptomatic people may be warranted for those exposed to fresh water in endemic areas.
Schistosomiasis is diagnosed and parasite burden is estimated by microscopic examination of stool or urine ( S. haematobium ) for eggs. Repeated examinations using concentration techniques may be necessary. Geography is a primary determinant of species, so the location of exposure should be communicated to the laboratory. If the clinical picture suggests schistosomiasis but no eggs are found after repeated examination of urine or feces, intestinal or bladder mucosa can be biopsied to check for characteristic granulomas around embedded eggs.
Tests for schistosomal antigens or DNA in blood, urine, or stool are particularly useful for schistosome eradication programs and in returning travelers with suspected infection. Most antigen detection tests are quantitative and antigen levels are correlated to parasite burden. Some antigen tests, such as the commercially available urine dipstick for S. mansoni , are qualitative.
Serologic tests are sensitive and specific for infection, but do not provide information about worm burden, clinical status, or prognosis and do not distinguish active from resolved infection. Antibody tests thus are most useful for detecting infection in returning travelers and not helpful in patients who are residents of endemic areas. With returning travelers, serologic tests should be done ≥ 6 to 12 weeks after the last exposure to fresh water to allow time for maturation of the schistosomes into adults and for development of antibodies.
Hepatosplenic schistosomiasis can be diagnosed by finding eggs in stool, intestinal tissue, or liver samples taken for biopsy with variable sensitivity as egg shedding can be intermittent in such patients. Typically, liver blood tests are normal. Ultrasonography may show periportal fibrosis and splenomegaly.
Neuroschistosomiasis is diagnosed if there is infection at an extraneural site along with clinical and radiographic evidence of neurologic involvement. Schistosomes in biopsied central nervous system lesions, and/or a positive antibody test or polymerase chain reaction in cerebrospinal fluid are also diagnostic.

Treatment of Schistosomiasis
S. haematobium , S. mansoni , and S. intercalatum ; 20 mg/kg 3 times a day for S. japonicum and S. mekongi
If eggs are present at the time of diagnosis, follow-up examination 1 to 2 months after treatment is suggested to help confirm cure. Treatment is repeated if eggs are still present.
Treatment of acute schistosomiasis (Katayama fever)
Patients with eggs in stool or urine at the time acute or chronic schistosomiasis is diagnosed should be examined for living eggs 1 to 2 months after treatment. An experienced microscopist can distinguish viable eggs from empty shells based on the presence of living miracidium. Retreatment is indicated if viable eggs are present.
Prevention of Schistosomiasis
Scrupulously avoiding contact with contaminated fresh water prevents schistosomiasis.
Schistosomiasis is not transmitted by swallowing contaminated water; however, mouth and lip contact with contaminated water could lead to infection.
Fresh water used for bathing should be boiled for at least 1 minute and then cooled before bathing. However, water that has been held in a storage tank for at least 1 to 2 days should be safe without boiling.
People who are accidentally exposed to possibly contaminated water (eg, by falling into a river) should vigorously dry off with a towel to attempt to remove any parasites before they penetrate the skin.
The sanitary disposal of urine and feces reduces the likelihood of infection.
Adult residents of endemic areas are more resistant to reinfection than children, suggesting the possibility of acquired immunity.
Vaccine development is under way.
Schistosoma is the only trematode that invades through the skin; over 230 million people are infected worldwide.
When cercariae penetrate the skin, they lose their forked tail and become schistosomula, which travel through the bloodstream to the liver, where they mature; as adults, they migrate to their ultimate home in the intestinal veins or the venous plexus of the genitourinary tract.
Ova in the liver trigger a granulomatous reaction that can lead to fibrosis and portal hypertension, resulting in splenomegaly, esophageal varices, and hematemesis.
Organisms in the intestine can cause bloody diarrhea, and organisms in the bladder can cause hematuria and chronic cystitis.
To prevent infection, avoid contact with fresh water in endemic areas.
Dermatitis Caused by Avian and Animal Schistosomes
Cercariae of Schistosoma species that infect birds and mammals other than humans can penetrate human skin. Although the organisms do not develop in humans, humans may become sensitized and develop pruritic maculopapular or vesicular skin lesions at the site of penetration. Skin lesions may be accompanied by a systemic febrile response that runs for 5 to 7 days and resolves spontaneously.
Cercarial dermatitis occurs worldwide. In North America, ocean-related schistosome dermatitis (clam digger's itch) occurs on all Atlantic, Gulf, Pacific, and Hawaiian coasts. It is common in muddy flats off Cape Cod. Freshwater schistosome dermatitis (swimmer's itch) is common in the Great Lakes region.
Diagnosis of cercarial dermatitis is based on clinical findings. Most cases do not require medical attention.
Treatment of cercarial dermatitis is symptomatic with cool compresses, baking soda, or antipruritic lotions. Topical corticosteroids can also be used.

Copyright © 2024 Merck & Co., Inc., Rahway, NJ, USA and its affiliates. All rights reserved.
- Cookie Preferences
- Search Menu
- Sign in through your institution
- Advance articles
- Editor's Choice
- Supplement Archive
- Editorial Commentaries
- Perspectives
- Cover Archive
- IDSA Journals
- Clinical Infectious Diseases
- Open Forum Infectious Diseases
- Author Guidelines
- Open Access
- Why Publish
- IDSA Journals Calls for Papers
- Advertising and Corporate Services
- Advertising
- Reprints and ePrints
- Sponsored Supplements
- Branded Books
- Journals Career Network
- About The Journal of Infectious Diseases
- About the Infectious Diseases Society of America
- About the HIV Medicine Association
- IDSA COI Policy
- Editorial Board
- Self-Archiving Policy
- For Reviewers
- For Press Offices
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Results and discussion, conclusions, supplementary data.
- < Previous
The Human Blood Fluke, Schistosoma mansoni , Harbors Bacteria Throughout the Parasite's Life Cycle
G. R. and C. C. are senior authors and contributed equally to this work.
- Article contents
- Figures & tables
Fabio Formenti, Alba Cortés, Michela Deiana, Susannah Salter, Julian Parkhill, Matt Berriman, Gabriel Rinaldi, Cinzia Cantacessi, The Human Blood Fluke, Schistosoma mansoni , Harbors Bacteria Throughout the Parasite's Life Cycle, The Journal of Infectious Diseases , Volume 228, Issue 9, 1 November 2023, Pages 1299–1303, https://doi.org/10.1093/infdis/jiad288
- Permissions Icon Permissions
While symbiotic relationships between invertebrates and bacteria have been extensively described, studies of microbial communities inhabiting parasitic worms remain scarce. Exploring the microbiota associated with helminths responsible for major infectious diseases will inform on parasite biology, host-pathogen interactions, and disease pathophysiology. We investigated the presence of microorganisms inhabiting tissues of the human parasite Schistosoma mansoni . In situ hybridization using a pan-bacterial 16S rRNA gene probe revealed bacteria colonizing key developmental stages that were successfully removed after antibiotic treatment of live parasites. Understanding the composition and function of the S. mansoni -associated microbiota may lead to the development of novel microbiome-targeting control strategies
Schistosomiasis is a neglected tropical disease affecting >250 million people worldwide [ 1 ], caused by Schistosoma blood flukes. The parasite life cycle involves both vertebrate, including human, and invertebrate hosts (ie, freshwater snails) [ 1 ]. Humans become infected when free-living larvae (cercariae), released in freshwater from Biomphalaria snails, penetrate the skin and become schistosomula. Schistosomula migrate within the circulatory system via the lungs to the liver vasculature, where they develop and pair up before migrating to the mesenteric veins (in the case of Schistosoma mansoni ). Four weeks post-infection, female worms begin to lay eggs that traverse the lining of the mesenteric vessels, migrate through the intestinal wall to the lumen and are excreted with host feces. In fresh water, the eggs hatch ciliated miracidia that infect the snail, undergo asexual multiplication, and develop from primary and secondary sporocysts to cercariae. The pathophysiology of schistosomiasis is mainly driven by parasite eggs becoming trapped in the liver and intestines, promoting inflammation, granuloma formation, and fibrosis [ 1 ]. Control of schistosomiasis in endemic areas has long relied on the administration of a single drug, that is praziquantel, delivered in mass drug administration programs [ 1 ]. However, praziquantel is active only against adult worms, reinfections are common, and tissue lesions persist after treatment. The reliance on a single drug is likely to lead to the emergence of drug-resistant parasites [ 1 ]; thus, new drugs and eventually vaccines are desperately needed.
Fundamental knowledge of schistosome biology and interactions with their hosts may reveal exploitable vulnerabilities that could be targeted in the development of novel and sustainable control strategies. One emerging area of interest focuses on the occurrence of communities of microorganisms that inhabit the parasitic worm, collectively known as the worm-associated microbiota [eg,. 2 ]. Microorganisms closely affiliated with parasitic worms have been described in filarial nematodes and identified in the reproductive tissues of some digenean trematodes [eg, 2 ]. To date, a single study demonstrated that the tegument and gastrodermis of Schistosoma japonicum adult worms are inhabited by populations of bacteria whose functions remain unknown [ 3 ]. While this preliminary evidence is promising, determining whether schistosome-associated bacteria represent a potential target for intervention relies on the acquisition of additional knowledge of their location and propagation throughout the parasite life cycle. In this study, we applied fluorescence in situ hybridization (FISH) to explore the occurrence of S. mansoni -associated bacteria throughout key developmental stages. We detected a strong 16S rRNA signal associated to the apical/lateral glands of eggs and miracidia, to the acetabular glands and oral/ventral suckers of cercariae, and to the gut and tegument of adult worms. Our findings indicate that propagation of bacteria associated with S. mansoni may occur vertically via the eggs.
The life cycle of S. mansoni (NMRI strain) was maintained at the Wellcome Sanger Institute. The Animal Welfare and Ethical Review Body of the Wellcome Sanger Institute approved all regulated animal procedures conducted under the Home Office Project License P77E8A062.
S. mansoni developmental stages were obtained from experimentally infected outbred TO mice as follows: adult worms were collected by portal perfusion; eggs were isolated by collagenase-digestion of infected livers; and miracidia hatched from eggs incubated in water under light [ 4 ]. Cercariae were recovered from experimentally infected Biomphalaria glabrata [ 4 ]. Specimens were fixed in 4% paraformaldehyde in 1× phosphate-buffered saline-0.3% Triton (PBS-T) overnight at 4°C, dehydrated in increasing concentrations of methanol in 1× PBS-T and stored in 100% methanol at −20°C until use. Fixed specimens were rehydrated, through a decreasing methanol concentration series, and paraffin embedded. For each specimen, four 4-µm thick sections were obtained; 1 section was stained with hematoxylin/eosin to assess parasite morphology, while the remaining 3 sections were subjected to FISH, using the KBI-60007 Tissue Digestion Kit I (Leica Biosystems) according to manufacturer's instructions. Briefly, sections were incubated for 1 hour at 70°C and deparaffinized in xylene for 10 minutes. Specimens were rehydrated in 100%, 85%, and 70% ethanol, for 3 minutes each, before washing in water for 1 minute at room temperature (RT). Sections were placed in 0.01 M sodium citrate at 96°C for 15 minutes and incubated for 5 minutes at RT in approximately 200 µL of pepsin solution. Thereafter, specimens were washed in water for 1 minute and in 2× saline-sodium citrate (SSC) buffer for 5 minutes before dehydration with 1-minute washes in 70%, 85%, and 100% ethanol. Sections were incubated with 10 µL of a 100 µM fluorescent probe (universal bacterial probe or control nonsense probe) for 2 hours at 46°C in total darkness. An additional section, incubated with water under the same conditions, was included as control. The Eubacteria probe EUB338 (Eurofins Genomics) was used that includes part of the 16S rRNA gene, double-labelled with ATTO 594 (5′-ATTO594-GCTGCCTCCCGTAGGAGT-3′) [ 5 ]. The nonsense probe, Non-EUB338 (Eurofins Genomics), with a complementary sequence to the EUB338 probe and the same fluorophore (ATTO-594) was used as a negative control for non-specific binding [ 5 ]. Following probe incubation, sections were washed in 200 µL of 2× SCC/0.3% Igepal lysis buffer for 2 minutes at RT, followed by a wash with 0.4× SCC/0.3% Igepal lysis buffer for 2 minutes at 72°C. Samples were treated with 0.1% Sudan Black B (w/v) (Sigma-Aldrich) for 20 minutes, to reduce autofluorescence , then dehydrated with 1-minute washes in 70%, 85%, and 100% ethanol, before mounting in mounting medium with DAPI (4′, 6-diamidino-2-phenylindole). Fluorescence was evaluated using a Leica SP5 AOBS confocal microscope, with a 40× oil immersion HC PL APO CS2-NA 1.3 objective.
To evaluate the susceptibility of S. mansoni -associated bacteria to antibiotics, freshly collected mixed-sex adult worms were cultured at 37°C, 5% CO 2 in Dulbecco’s Modified Eagle’s Medium (DMEM) complemented with 10% fetal bovine serum, in the presence or absence of a wide-spectrum antibiotic cocktail consisting of 1000 U/mL penicillin, 1000 μg/mL streptomycin, and 100 μg/mL kanamycin (final concentrations). Culture media and antibiotic cocktail were replaced daily for 3 days. Aliquots of approximately 5 worm pairs from the antibiotic-treated or control group were collected daily for 3 days and processed for FISH.
To explore the occurrence of Schistosoma -associated bacteria, we employed FISH using a pan-bacterial 16S rRNA gene-specific probe that revealed a strong signal in miracidia, cercariae, eggs, and adult worms. Careful microscopical examination of the specimens returning positive 16S rRNA signals did not reveal any structural damage that may have led to bacterial contamination, thus providing confidence in the robustness of our data. In the miracidium, the signal was localized to the apical and lateral glands ( Figure 1 A and 1 B , and Supplementary Figure 1 ). These glands contain droplets and electrodense granular material secreted during invasion of the snail tissue [ 7 ]. These secretions consist of proteases, including acidic endopeptidases, and other excretory-secretory products involved in tissue invasion and immune modulation [ 8 ]. Cercariae also returned a strong 16S rRNA signal in correspondence to the pre- and postacetabular glands ( Figure 1 C and 1 D , and Supplementary Figure 1 ). These glands open to the anterior tip of the cercaria head through ducts packed with secretory bodies that contain proteases involved in skin degradation, cercaria-schistosomulum transformation, and immune-evasion mechanisms [ 9 ]. No molecules have thus far been identified in the secretory glands of miracidia or cercariae that may originate from bacteria colonizing these organs. The origin, composition, and functional roles of bacteria (or their metabolites) inhabiting the secretory glands of schistosome larvae during host penetration and infection establishment remain unknown. Eggs of S. mansoni were classified according to their embryonic development [ 9 ]. A strong 16S rRNA signal was detected within the mature embryo, with a fluorescence pattern and location similar to the one identified in miracidia ( Figure 1 E , 1 F , and 1 M , and Supplementary Figure 1 ), while no 16S rRNA signal could be detected in immature eggs and underdeveloped embryos ( Figure 1 K and 1 L ) (Fisher exact test; n = 62; P = .00029; details provided in legend to Figure 1 ).
![essay on blood flukes Fluorescence in situ hybridization reveals bacteria in Schistosoma mansoni. Bacteria-specific fluorescent signal (red) in representative specimens of S. mansoni developmental stages and corresponding negative (ie, irrelevant probe) controls: miracidia (A and B, n = 10); cercariae (C and D, n = 10); eggs (E and F, n = 12 mature eggs); male adult worms (G and H, n = 10); and female adult worms (I and J, n = 10). Blue, DAPI-stained nuclei. Yellow arrow, g, gut; white arrow, t, tegument. For each developmental stage, the number of parasites returning a positive fluorescent signal, out of a total number of screened specimens, is indicated. K–M, Diagrams (left) and representative images (right) of immature (K), intermediate (L), and mature (M) eggs, respectively. Blue, DAPI-stained nuclei. For statistical analyses, eggs were classified as immature (Jurberg scores 0–5) or mature (Jurberg scores 6, 7) [6]. The statistical significance of the association between egg development and detection of 16S rRNA signal was tested by Fisher exact test performed on a group of 62 randomly selected eggs (n = 62; P value = .00029). Scale bars as indicated.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/228/9/10.1093_infdis_jiad288/1/m_jiad288f1.jpeg?Expires=1734478804&Signature=NX2iQqIKeuZRN-fPYbqgjeHB6R-7NL5jqEco6af0tnYRrHN7XXuMbrVcTOPKPUfffsDQG4xRhm3-hTb33EbagvqmCok613SzsKyAxSy8LqGizXv4qse4ZYAgav8lWubrL2iDULfNopClyOnhPUNc06ItvgpLIwpS8ddviBKYjcY3jQUkWeh1yCuxMcBxW7C9ts~rifqFlXrChJd-32myo8bz1Lb5i2rlOiTsEmzeTWhC-wSIIh8vZ2a2Ymwkr86ON2284IOQFn8mjdyjOPkxmJsPSwAMy4QuV~41cXC6kTH1-m~-Nu6Trk6SAUkWhQ~oVVe2INjgdHicQXJz-Vz18w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Fluorescence in situ hybridization reveals bacteria in Schistosoma mansoni . Bacteria-specific fluorescent signal (red) in representative specimens of S. mansoni developmental stages and corresponding negative (ie, irrelevant probe) controls: miracidia ( A and B , n = 10); cercariae ( C and D , n = 10); eggs ( E and F , n = 12 mature eggs); male adult worms ( G and H , n = 10); and female adult worms ( I and J , n = 10). Blue, DAPI-stained nuclei. Yellow arrow, g, gut; white arrow, t, tegument. For each developmental stage, the number of parasites returning a positive fluorescent signal, out of a total number of screened specimens, is indicated. K–M , Diagrams (left) and representative images (right) of immature ( K ), intermediate ( L ), and mature ( M ) eggs, respectively. Blue, DAPI-stained nuclei. For statistical analyses, eggs were classified as immature (Jurberg scores 0–5) or mature (Jurberg scores 6, 7) [ 6 ]. The statistical significance of the association between egg development and detection of 16S rRNA signal was tested by Fisher exact test performed on a group of 62 randomly selected eggs (n = 62; P value = .00029). Scale bars as indicated.
To the best of our knowledge, our study is the first to describe the occurrence of bacteria in mature eggs, miracidia, and cercariae of schistosomes. Distantly related trematodes are known to be permanently colonized by obligate intracellular Neorickettsia bacteria [ 2 ], that are occasionally transferred to vertebrate host tissues over the course of infection, causing severe diseases [ 10 ]. Neorickettsia spp. have been localized to different tissues of adult worms, including the vitelline cells of the liver fluke Fasciola hepatica [ 10 ]. This localization suggests vertical transmission of endosymbionts from adult worms to offspring via the eggs. We detected a strong 16S rRNA signal lining the tegument and gastrodermis of male ( Figure 1 G and 1 H , and Supplementary Figure 2 ) and female worms ( Figure 1 I and 1 J , and Supplementary Figure 2 ), consistent with a recent study of adult S. japonicum [ 4 ]. Similar to Gobert et al [ 3 ], we did not detect a 16S rRNA signal in the ovary and vitellaria of adult females, or testes of male S. mansoni ( Supplementary Figure 2 ). Nevertheless, the observation of a strong 16S rRNA signal in mature eggs, coupled with the lack of signal in immature eggs and underdeveloped embryos, points toward a technical, rather than biological, explanation. The small diameter of Schistosoma egg-shell pores [ 11 ] makes acquisition of bacterial cells from the external environment highly unlikely; thus, we hypothesize that schistosome reproductive tissues may harbour a small bacterial mass below the sensitivity threshold of our FISH assay. Future studies may address this hypothesis by, for example, monitoring bacterial 16S rRNA signal in developing adult reproductive tissues and throughout maturation of eggs collected from antibiotic-treated females.
To investigate the effect of antimicrobials on the S. mansoni -associated microbiota, we collected and cultured mixed-sex adult worms for 72 hours in the presence or absence of an ad hoc cocktail of broad-spectrum antibiotics. No overt effect on parasite survival or fitness in worms post-incubation was observed ( Supplementary Figure 3 ), and no 16S rRNA signal was detected in worms exposed to antibiotics for 48 and 72 hours compared to controls ( Figure 2 ). The marked reduction and subsequent elimination of fluorescent signal in antibiotic-treated adult worms further strengthens our hypothesis of the occurrence of S. mansoni -associated bacteria. Associations between adult schistosomes and both gram-positive and gram-negative bacteria have been described [ 12 ]. For instance, Salmonella paratyphi A was demonstrated to colonize the tegument of adult S. mansoni recovered from the bloodstream of a human patient [ 13 ]. A later study [ 14 ] showed that the interactions between adult schistosomes and Salmonella occur via adherence of bacterial pili to the worm tegument, mediated by mannose-like receptors. The authors further hypothesized that adult schistosomes allow multiplication of Salmonella in the mesenterial system, thus accounting for the protracted course of typhoid fever in coinfected patients [ 14 ]. Interestingly, experimental infection of adult S. mansoni with different groups of bacteria administered intravenously resulted in varying degrees of antischistosomal activity. Selected Enterobacteriaceae (eg, Escherichia , Salmonella, and Klebsiella ) rapidly colonized and multiplied within the schistosome coecum, resulting in worm death. Other genera (eg, Achromobacter and Flavobacterium ) led to a limited colonization and temporary decrease of egg output [ 15 ]. The mechanisms by which some bacteria killed adult S. mansoni have not yet been established. Nevertheless, non-exclusive hypotheses include (1) parasite muscle disruption; (2) mechanical tissue damage by large numbers of bacteria colonizing the coecum; (3) secretion of bacterial endotoxin; and (4) competition for essential nutrients and/or metabolites. The latter hypothesis implies that externally acquired bacteria may compete for space and nutrients with schistosome-associated bacteria, thus possibly leading to significant disturbances in the parasite microbial homeostasis.

Bacterial fluorescent signal reduction in antibiotic-treated parasites. Bacterial 16S rRNA fluorescent signal (red) in representative specimens of adult Schistosoma mansoni at 24, 48, and 72 hours after antibiotic treatment ( A , C, and E , respectively) and corresponding controls ( B , D, and F , respectively). Blue, DAPI-stained nuclei. For each time point post-antibiotic treatment, the number of parasites displaying a reduced fluorescent signal, out of a total number of screened specimens, is indicated. Yellow arrow, g, gut; white arrow, t, tegument. Scale bars as indicated.
We report the occurrence of bacteria associated with different developmental stages of S. mansoni . Our study sets the stage for functional characterization of these microbial communities using metagenomics approaches. However, capturing the structure of small bacterial communities colonizing helminth tissues is challenging, mainly due to the underrepresentation of target bacteria, compared with worm cells and contaminating environmental bacteria. Our initial efforts to characterize bacteria colonizing S. mansoni using metagenomics resulted in overrepresentation of contaminant sequences, which prevented us from drawing robust conclusions. Pre-extraction and/or post-extraction methods to enrich for microbial DNA prior to library construction, and spike-in standards added to S. mansoni specimens, may assist with overcoming these challenges. Insights into the structure and function of the schistosome-associated microbiota may ultimately lead to the discovery and development of novel strategies for parasite control.
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Author contributions. F. F., A. C., G. R., and C. C. conceived the study and obtained funding. F. F., M. D., and G. R. performed the experiments and analyzed the data. F. F., G. R., and C. C. wrote the first draft. A. C., S. S., J. P., and M. B. provided comments to the draft. All authors read and approved the final draft.
Acknowledgments . The authors express their gratitude to Simon Clare, Catherine McCarthy, Katherine Harcourt, Cordelia Brandt, and Charlotte Tolley for technical assistance with rodent experimental infections and collection of parasite specimens.
Disclaimer. The funders did not participate in the design and conduct of the study, in the collection, management, analysis, and interpretation of the data, in the preparation, review or approval of the final draft, or in the decision to submit the manuscript for publication.
Financial support . This work was supported by the IRCCS Sacro Cuore Don Calabria, Verona Italy (MPhil scholarship to F. F.); UK Research and Innovation (UKRI; Future Leaders Fellowship to G. R.); and the Ministry of Health Italy Fondi Ricerca Corrente-L3, P2 to IRCCS Sacro Cuore Don Calabria Hospital, Verona, Italy. C. C.’s laboratory is supported by the UKRI and the Isaac Newton Trust. Funding to pay the Open Access publication charges for this article was provided by the University of Cambridge.
Potential conflicts of interest . All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Mutapi F , Maizels R , Fenwick A , Woolhouse M . Human schistosomiasis in the post mass drug administration era . Lancet Infect Dis 2017 ; 17 : e42 – e8 .
Google Scholar
Jenkins TP , Brindley PJ , Gasser RB , Cantacessi C . Helminth microbiomes—a hidden treasure trove? Trends Parasitol 2019 ; 35 : 13 – 22 .
Gobert GN , McManus DP , McMullan G , et al. Adult schistosomes have an epithelial bacterial population distinct from the surrounding mammalian host blood . PLoS One 2022 ; 17 : e0263188 .
Mann VH , Morales ME , Rinaldi G , Brindley PJ . Culture for genetic manipulation of developmental stages of Schistosoma mansoni . Parasitology 2010 ; 137 : 451 – 62 .
Wallner G , Amann R , Beisker W . Optimizing fluorescent in situ hybridization with rRNA-targeted oligonucleotide probes for flow cytometric identification of microorganisms . Cytometry 1993 ; 14 : 136 – 43 .
Jurberg AD , Gonçalves T , Costa TA , et al. The embryonic development of Schistosoma mansoni eggs: proposal for a new staging system . Dev Genes Evol 2009 ; 219 : 219 – 34 .
Pan SC . The fine structure of the miracidium of Schistosoma mansoni . J Invertebr Pathol 1980 ; 36 : 307 – 72 .
Hambrook JR , Hanington PC . Immune evasion strategies of schistosomes . Front Immunol 2021 ; 11 : 624178 .
Dorsey CH , Stirewalt MA . Schistosoma mansoni : fine structure of cercarial acetabular glands . Exp Parasitol 1971 ; 30 : 199 – 214 .
McNulty SN , Tort JF , Rinaldi G , et al. Genomes of Fasciola hepatica from the Americas reveal colonization with Neorickettsia endobacteria related to the agents of Potomac horse and human Sennetsu fevers . PLoS Genet 2017 ; 13 : e1006537 .
Race GJ , Michaels RM , Martin JH , Larsh JE Jr , Matthews JL . Schistosoma mansoni eggs: an electron microscopic study of shell pores and microbarbs . Proc Soc Exp Biol Med 1969 ; 130 : 990 – 2 .
LoVerde PT , Amento C , Higashi GI . Parasite-parasite interaction of Salmonella typhimurium and Schistosoma . J Infect Dis 1980 ; 141 : 177 – 85 .
Young SW , Higashi G , Kamel R , el-Abdin AZ , Mikhail IA . Interaction of salmonellae and schistosomes in host-parasite relations . Trans R Soc Trop Med Hyg 1973 ; 67 : 797 – 802 .
Melhem RF , LoVerde PT . Mechanism of interaction of Salmonella and Schistosoma species . Infect Immun 1984 ; 44 : 274 – 81 .
Ottens H , Dickerson G . Studies on the effects of bacteria on experimental schistosome infections in animals . Trans R Soc Trop Med Hyg 1972 ; 66 : 85 – 107 .
Author notes
- antibiotics
- in situ hybridization
- fluorescent in situ hybridization
- communicable diseases
- invertebrates
- life cycle stages
- rna, ribosomal, 16s
- schistosomatidae
- schistosoma mansoni
- developmental stages
- microorganisms
- microbial colonization
- host-pathogen interactions
Supplementary data
Email alerts, more on this topic, related articles in pubmed, citing articles via, looking for your next opportunity.
- X (formerly Twitter)
- Recommend to your Library
Affiliations
- Online ISSN 1537-6613
- Print ISSN 0022-1899
- Copyright © 2024 Infectious Diseases Society of America
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

IMAGES
VIDEO