An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
NICE Medicines and Prescribing Centre (UK). Medicines Optimisation: The Safe and Effective Use of Medicines to Enable the Best Possible Outcomes. Manchester: National Institute for Health and Care Excellence (NICE); 2015 Mar. (NICE Guideline, No. 5.)


Medicines Optimisation: The Safe and Effective Use of Medicines to Enable the Best Possible Outcomes.
8 medication review, 8.1. introduction.
Medication review, as an overarching term, has been considered to be an important intervention for many years. Medication review can have several different meanings. It could be a review of medicines carried out every day when a prescriber sees a patient and there is a decision to prescribe or stop a medicine, or a multidisciplinary medication review, with the patient (and their family members or carers where appropriate) present, using a comprehensive and structured approach supported by the patient's full medical records. Medication reviews are carried out in people of all ages.
In 2001, the National Service Framework for older people included a milestone stating that ‘all people over 75 years should normally have their medicines reviewed at least annually and those taking 4 or more medicines should have a review 6 monthly’. The National Service Framework (NSF) did not provide information as to what this medication review should entail or how it should be carried out. To support the implementation of this milestone, it was incorporated into the General Medical Services Quality and Outcomes Framework (QOF) from 2006 until 2013.
Room for review – a guide to medication review: the agenda for patients, practitioners and managers was published by the Taskforce on Medicines Partnership, The National Collaborative Medicines Management Services programme in 2002. This document suggested key principles for the process of medication review:
- All patients should have a chance to raise questions and highlight problems about their medicines.
- Medication review seeks to improve or optimise impact of treatment for an individual patient.
- The review is undertaken in a systematic way, by a competent person.
- Any changes resulting from the review are agreed with the patient.
- The review is documented in the patient's notes.
- The impact of any change is monitored.
This document, along with a subsequent National Prescribing Centre document A guide to medication review (2008), aimed to clarify the different types of medication review. Box 1 summarises the different types of medication review, adapted from Room for Review (2002) and A guide to medication review (2008).
Different types of medication review.
In 2005, the National Service Framework for long-term conditions recommended in its quality requirement 1, that ‘people have timely, regular medication review’. Again, there were no specific details about what this medication review entailed.
The National Prescribing Centre issued A guide to medication review (2008), which gave further guidance for medication reviews to commissioners and providers of services. This document further emphasised the need to involve patients in discussions about their medicines and stated that patients ‘had a varied experience of the review and varied perceptions of its benefits’.
In 2013, the GMC issued updated guidance for Good practice in prescribing and managing medicines and devices . The updated guidance provides more detailed advice on how to comply with the principles outlined in the GMC document Good medical practice (2013). The guidance considers the process of reviewing medicines, and suggests that reviewing medicines is particularly important for patients who may be at risk, who are frail or who have multiple illnesses (which may increase polypharmacy ). It is also important for those patients who are taking medicines with potentially serious or common side effects (including high-risk medicines ) or those taking controlled drugs or other medicines that may be abused or misused. Reviewing medicines that require regular monitoring or blood tests is also important. The guidance also considers the role that pharmacists can play in medication review to ‘help improve safety, efficacy and adherence in medicines use, for example by advising patients about their medicines and carrying out medicines reviews’.
Over the years the process of medication review has evolved, but it is not a new approach. It can be carried out in a wide range of settings and the type and depth of medication review carried out varies. The type of health professionals who carry out medication reviews has also changed, with the changing medical model of prescribing, supplying and administering medicines (see section on medicines-related models of care). Regardless of which health professional is carrying out the medication review, there appears to be variation in how and when reviews are carried out.
Furthermore it is important to consider the cost of medication reviews to the NHS. Cost effectiveness analyses of medication review have been carried out over a number of years, often alongside RCTs or other clinical studies. Costing studies for medication reviews have been carried out in several different countries. Such analyses calculate the costs of performing medication reviews and some aim to determine the subsequent costs and health benefits of the intervention.
Medicines use reviews are different from medication reviews because the pharmacist carrying out the review does not have access to the patient's medical records. A medicines use review can complement a medication review. Information about medicines use reviews is available on the Royal Pharmaceutical Society website . In addition to medicines use reviews, community pharmacists also provide a new medicines service to support people with long-term conditions who are newly prescribed a medicine to improve adherence. Several other NICE guidelines recommend carrying out a medication review. Again in many of the guidelines details about how the medication review should be carried out, who should be involved and the overall process are not specified.
For the purpose of this guideline, when the term medication review is used, this is ‘a structured, critical examination of a person's medicines with the objective of reaching an agreement with the person about treatment, optimising the impact of medicines, minimising the number of medication-related problems and reducing waste’ ( National Prescribing Centre 2008 ).
Medication reviews can be offered at different levels, by different health professionals working in different settings. Therefore the aim of this review question was to review whether the evidence for a full, structured medication review led to a reduction in suboptimal use of medicines and medicines-related safety incidents.
8.2. Review question
What is the effectiveness and cost effectiveness of medication reviews to reduce suboptimal use of medicines and medicines-related patient safety incidents, compared to usual care?
8.3. Evidence review
A systematic literature search was conducted (see appendix C.1 ), which identified 1565 references. The review protocols identified the same parameters for the review question on medication review and medicines reconciliation. Therefore a single search was carried out. After removing duplicates, the references were screened on their titles and abstracts and each included study was identified as being relevant for inclusion for the review question on medicines reconciliation or medication review. Sixty-five references were obtained and reviewed against the inclusion and exclusion criteria as described in the review protocol for medication review ( appendix C2.4 ).
Overall, 52 studies were excluded because they did not meet the eligibility criteria. A list of excluded studies and reasons for their exclusion is provided in appendix C5.4 .
Thirteen studies met the eligibility criteria and were included. In addition, 7 systematic reviews of studies ( RCTs and observational) were identified. The references included in these systematic reviews were also screened on their titles and abstracts, to identify any further studies that met the eligibility criteria. Fifteen additional studies were included.
All the included 28 studies were RCTs investigating the effect of medication reviews compared with usual care (see appendix D1.4 evidence tables for details). The studies were carried out in an adult population; there were no studies identified that looked at medication reviews in children. Twelve of the studies targeted medication reviews for patients with long-term conditions such as hypertension, angina, asthma, hyperlipidaemia, diabetes, coronary heart disease, arthritis and osteoporosis. Twenty-one RCTs looked at pharmacist-led medication review, 6 RCTs looked at multidisciplinary team-led medication review and 1 RCT looked at physician-led medication review.
The studies were quality assessed using the NICE methodology checklists for RCTs (see NICE guidelines manual 2012 ). Appraisal of the quality of the study outcomes was carried out using GRADE.
See appendix D1.4 for evidence tables summarising included studies.
See appendix D2.4 for GRADE profiles.
There was some pooling of studies, although this was limited because the outcomes measures used differed and the follow-up periods reported varied between the studies.
Mean differences were calculated for continuous outcomes and odds ratios for binary outcomes, as well as the risk ratios for dichotomous data. When a meta-analysis was possible, a forest plot was presented (see appendix D2.4 ).
Summary of included studies.
8.4. Health economic evidence
Summary of evidence.
A systematic literature search was undertaken ( appendix C.1 ) to identify cost effectiveness studies comparing medication reviews to reduce the suboptimal use of medicines. This search identified 1507 results, of which 1480 records were excluded following screening of their titles and abstracts. The full papers of 27 studies were assessed for relevance against the inclusion and exclusion criteria. Sixteen studies did not meet the inclusion criteria, the reasons for which are listed in appendix C.6.4 . A further 3 studies were identified as relevant from the clinical evidence review, resulting in a total of 14 studies meeting the inclusion criteria.
The studies meeting the inclusion criteria were quality assessed using the NICE quality assessment checklists for cost effectiveness studies. Following this, 4 studies were judged not applicable to the guidance because better quality studies (considering health-related quality of life), more relevant to a UK NHS setting, had been identified. An additional study ( Burns et al. 2000 ) was judged by the GDG to be not relevant to the current UK NHS because of the age of the study. This study was a cost comparison study with a short time horizon and therefore evidence more relevant to the decision problem had already been included. These 5 studies judged not applicable to the guidance were excluded from further analysis. Three further studies were considered to have very serious limitations and were excluded on that basis.
Table 22 shows the economic evidence profile based on the 6 included studies. These studies were judged to be partially relevant to the guidance. The evidence was of variable quality: 4 of the 6 included studies were of low quality and 2 were of high quality. The economic evidence on medication review was limited to review by pharmacists in different settings with no analysis beyond a 12-month time horizon ( appendix E1.4 ).
Economic evidence profile – medication reviews.
A health economic model was not pursued in this area because of the lack of data on medication reviews carried out by health professionals other than pharmacists. There is already an evidence base of published cost–utility studies relating to pharmacist review, so the GDG judged that a de novo model in this area would not help their recommendations. The limited data availability relating to other types of medication review meant that a robust economic model could not be constructed. To aid the GDG discussions a simple costing analysis was undertaken to compare the costs of medication reviews undertaken by a variety of health professionals. A summary of this is provided after table 23 .
Estimate of cost per medication review delivered.
Summary of economic modelling
A summary of the simple cost analysis carried out for this area of the guidance is provided. See appendix F for a full report of the cost analysis carried out for this clinical guideline topic.
Simple costing calculations were carried out to provide the GDG with information around the cost per medication review undertaken dependent upon the health professional delivering the review. These are displayed in table 23 . The length of time utilised for each medicine review was estimated by the GDG and various scenarios are displayed. Health professional costs were sourced from the Personal Social Services Research Unit (PSSRU) ( PSSRU, 2013 ).
A variety of cost options are displayed, which include salary costs only, PSSRU unit cost per health professional and PSSRU unit cost per hour of health professional contact with patients, for consideration by the GDG. It is important to note that an NHS and PSS perspective should be taken for all NICE guidance ( NICE, 2012 ). The costs provided in table 23 are limited in that they provide no information on the quality and impact of the review, nor the long term cost savings resulting from the review.
8.5. Evidence statements
Clinical evidence.
Low-quality evidence from 10 RCTs showed no significant difference in mortality between patients receiving medication review or usual care. The patient populations in the 10 RCTs were people with a mean age of 65 years or over, and who had medication reviews carried out by pharmacists (8 RCTs), a physician (1 RCT) or a clinical pharmacologist (1 RCT).
Low-quality evidence from 2 RCTs pooled together to show the effect of pharmacist-led medication review on targeted hypertensive patients showed that there was a significant change in mean blood systolic pressure in the intervention group compared with control group to meet the target blood pressure.
Low-quality evidence from 1 RCT showed that pharmacist-led medication reviews in a population with a mean age of 65 years significantly increased the percentage of intervention patients achieve target blood pressure compared with usual care. The same RCT showed a significant improvement in low-density lipoprotein (LDL) levels, reaching target INR with anticoagulation and improving diabetes control by meeting HbA 1c targets with medication reviews.
Moderate- and low-quality evidence from 2 RCTs showed that medication reviews compared with usual care in a population with a mean age of 60 years reported no significant differences in the following clinical outcomes (as reported in the studies): proportion of patients prescribed cardiovascular medicines for secondary prevention, 5-year risk of cardiovascular death score, target lipid levels or reduction in LDL levels and changes in cardiovascular risk factors.
Low-quality evidence from 1 RCT showed that medication reviews in patients with asthma significantly improved the optimisation of asthma medicines, asthma severity and inhaler technique, but there was no improvement in spirometry results compared with usual care.
Moderate-quality evidence from 1 RCT showed that medication review significantly reduced the need for rescue medicine and night-time awakenings in patients with asthma. However, it did not improve the peak expiratory flow, asthma control test (ACT) scores or occurrence of severe exacerbations of asthma.
Moderate-quality evidence from a meta-analysis of 2 RCTs showed that medication reviews in a population with a mean age of 84 years significantly reduced the number of falls. One low-quality evidence RCT reported that medication reviews did not significantly reduce the number of fractures in the elderly. Another moderate-quality evidence RCT carried out in a population with a mean age of 68 years showed that medication reviews when compared with usual care significantly improved pain scores, pain severity score, arthritis self-efficacy pain scale scores and clinical response within the first 3 months of the intervention.
Low-quality evidence from 1 RCT showed that medication reviews did not significantly improve the reporting of adverse drug events compared with usual care.
Moderate-quality evidence from 1 RCT showed no significant differences in the number of adverse drug events per 1000 days between the groups that received medication review or usual care.
Moderate-quality evidence from 2 RCTs showed that medication reviews identified more medicines-related problems compared with usual care, but there was no significant difference between the 2 groups. In 1 RCT, medication reviews significantly reduced the mean number of potential medicines-related problems from baseline to endpoint compared with usual care. Another RCT identified medicines-related problems in 71% of patients who received usual care before having a medication review.
Moderate-quality evidence from 1 RCT in an elderly population showed that medication reviews significantly reduced the prescribing of inappropriate medicines compared with usual care.
Moderate-quality evidence from 1 RCT showed that medication reviews in patients with a mean age of 65 years reduced the Medication Appropriateness Index (MAI) scores in all 10 domains compared with usual care. However, the statistical significance of the results was not reported.
Low-quality evidence from 2 RCTs pooled together showed that medication reviews significantly reduced the MAI scores compared with usual care. One moderate-quality evidence RCT showed that medication reviews reduced the number of potentially inappropriate medicines per patient compared with usual care, but this was not significant. Moderate-quality evidence from 1 RCT showed that medication reviews identified more inappropriate medicines (as per Beers criteria) prescribed (although not significant) and also identified significantly more underused medicines (as per ACOVE criteria) compared with usual care.
Moderate-quality evidence from 1 RCT carried out in patients with dyslipidaemia showed that medication reviews reduced the number of patients being prescribed high-intensity statins compared with usual care. Moderate-quality evidence from 2 RCTs and low-quality evidence from 4 RCTs which reported on the mean number of medicines per patient for the study showed that there was no significant difference between the usual care and medication reviews after the follow-up period.
Moderate- and low-quality evidence from 2 RCTs showed that medication reviews significantly reduced the mean number of medicines prescribed compared with usual care. Moderate-quality evidence from 1 RCT reported a significantly smaller increase in the number of medicines prescribed to elderly patients who had medication reviews compared with usual care.
Low-quality evidence from 2 RCTs and moderate-quality evidence from 1 RCT showed that medication reviews significantly increased compliance compared with usual care. Moderate-quality evidence from 3 RCTs showed no significant difference in patient compliance between the groups that received medication reviews or those that received usual care.
Moderate-quality evidence from 2 RCTs and low-quality evidence from 1 RCT showed no significant difference in patient satisfaction between medication review and usual care. Moderate-quality evidence from 2 RCTs showed that medication reviews received significantly high patient satisfaction rates compared with usual care.
Moderate-quality evidence from 1 RCT showed no significant difference in the change in depression and anxiety scores for patients who received medication reviews or usual care. The same study showed that medication reviews significantly improved patients' clinical response to knee pain management (using the OMERACT-OARSI responder criteria) at 3 months compared with usual care. There was no significant difference at the end of the 12-month study.
Economic evidence
Partially applicable evidence from 2 studies with minor limitations, built on RCT data, suggests that pharmacist review is not cost effective, with ICERs above £50,000/QALY, compared with no intervention.
Partially applicable evidence from 4 studies with potentially serious limitations provided conflicting evidence, with 3 studies finding pharmacist review to be cost incurring and 1 finding it to be cost saving compared with no intervention.
No evidence was identified informing cost effectiveness of medication reviews by health professionals other than pharmacists.
8.6. Evidence to recommendations
Linking evidence to recommendations (LETR).
8.7. Recommendations and research recommendations
Medication review can have several different interpretations and there are also different types which vary in their quality and effectiveness. Medication reviews are carried out in people of all ages. In this guideline medication review is defined as ‘a structured, critical examination of a person's medicines with the objective of reaching an agreement with the person about treatment, optimising the impact of medicines, minimising the number of medication-related problems and reducing waste’. See also recommendation 33.
Consider carrying out a structured medication review for some groups of people when a clear purpose for the review has been identified. These groups may include:
- adults, children and young people taking multiple medicines ( polypharmacy )
- adults, children and young people with chronic or long-term conditions
- older people.
Organisations should determine locally the most appropriate health professional to carry out a structured medication review, based on their knowledge and skills, including all of the following:
- technical knowledge of processes for managing medicines
- therapeutic knowledge on medicines use
- effective communication skills.
The medication review may be led, for example, by a pharmacist or by an appropriate health professional who is part of a multidisciplinary team.
During a structured medication review, take into account:
- the person's, and their family members or carers where appropriate, views and understanding about their medicines
- the person's, and their family members' or carers' where appropriate, concerns, questions or problems with the medicines
- all prescribed, over-the-counter and complementary medicines that the person is taking or using, and what these are for
- how safe the medicines are, how well they work for the person, how appropriate they are, and whether their use is in line with national guidance
- whether the person has had or has any risk factors for developing adverse drug reactions (report adverse drug reactions in line with the yellow card scheme )
- any monitoring that is needed.
8.7.1. Research recommendation
To be read in conjunction with the NICE Research recommendations process and methods guide )
Uncertainties
This review question looked at the clinical and cost effectiveness of medication reviews to reduce the suboptimal use of medicines and medicines-related patient safety incidents, compared to usual care or other interventions. The systematic review provided no evidence for medication reviews carried out in children.
Uncertainties may be related to:
- clinical effectiveness and cost effectiveness of medication reviews carried out in children.
Reason for uncertainties
The searches did not identify any randomised controlled trials (RCTs) looking at medication reviews carried out in children. Although RCTs have been carried out in an adult population, the GDG found the outcomes used to measure the effectiveness were mixed. The GDG agreed that, although the principles of medication reviews carried out in adults can be applied to children, further research is needed in children because of the different levels of engagement and because it often needs parent or carer involvement where appropriate when carrying out the medication review. RCTs may not have been carried out in children because of ethical aspects.
The GDG also discussed and agreed that there was uncertainty about the following factors, which may affect the clinical and cost effectiveness of medication reviews:
- the type of medication review carried out (see section 8.1 )
- which health professional is carrying it out
- the frequency of medication review.
The GDG found that the above factors varied between the studies that were carried out in adults.
Key uncertainty
The key uncertainty is whether medication reviews can reduce the suboptimal use of medicines and medicines-related patient safety incidents compared with usual care or other interventions in children.
This uncertainty can be answered by conducting a study that will deliver good quality evidence, such as an RCT.
Recommendation
- Is a medication review more clinically and cost effective at reducing the suboptimal use of medicines and medicines-related patient safety incidents, compared with usual care or other interventions, in children?
The research should be carried out in children that use services where medication reviews can be carried out.
Study methodology can be based on other well-conducted RCTs that have been carried out in adults, the difference being the age of the population. Approval from ethics or other committees would be needed given the young age of the population. ‘Usual care’ or other interventions would be used as a comparator. ‘Usual care’ would need to be defined in the study. A follow-up period of 1–2 years or more would capture longer-term outcomes. The outcomes for this research question should be patient-centred and include suboptimal use of medicines, medicines-related patient safety incidents, patient-reported outcomes, clinical outcomes, medicines-related problems, health and social care resource use and cost effectiveness.
The study would need to take into account:
- the type of medication review carried out (see section 8.1 ); the study needs to outline a framework of the medication review to help guidance developers to see the process used; they would then be better able to decide if it would affect clinical effectiveness of the intervention
- the health professional carrying it out
- child, parent and carer involvement as this may affect some outcome measures, depending on their engagement level
- the frequency of medication review (this would impact on cost effectiveness of resource use).
The GDG recognised that the key focus of the medicines optimisation agenda is to make care person-centred. In line with this and to ensure the best use of NHS resources, the GDG agreed that research needs to be carried out in children to identify the benefit from them having medication reviews. There may be some longer-term gains with this approach, as from a young age the child would become more aware of the intervention, develop a relationship with the health professional and be encouraged to understand their medicines.
Research into this area will provide guidance to organisations who may want to, or already provide, medication reviews as part of their care and enable better use of resources (for example, health professional cost and time and health and social care resources). This information would be useful to commissioners who may consider whether or not to commission providers to carry out medication reviews.
Proposed format of research recommendations.
8.7.2. Research recommendation
To be read in conjunction with the NICE Research recommendations process and methods guide .
This review question looked at the clinical and cost effectiveness of medication reviews to reduce the suboptimal use of medicines and medicines-related patient safety incidents, compared to usual care or other interventions. The GDG found that the systematic review provided no economic evidence for medication reviews carried out by health professionals other than hospital or community pharmacists.
- cost effectiveness of medication reviews carried out in all settings, professional-led or carried out by a multidisciplinary team.
Reason for uncertainty
Most of the clinical and economic evidence identified related to medication reviews carried out by community or hospital pharmacists. There were randomised controlled trials (RCTs) that looked at the clinical effectiveness of medication reviews carried out by a doctor and by multidisciplinary teams (comprising a doctor, pharmacist and/or nurse), but there was no economic evidence for these. Therefore, the GDG was uncertain about the cost effectiveness of professional-led (other than hospital or community pharmacists) and multidisciplinary team medication reviews as no relevant data were available to feed into an economic model.
In addition, the included studies varied in quality and were carried out in Europe, Australia, Canada or the USA. The GDG was aware of the limitations relating to the applicability of some of these studies to the UK setting given the differences in healthcare systems and processes and populations.
The GDG discussed and agreed that the clinical and cost effectiveness of medication reviews would depend on several factors such as:
- type of health professional carrying it out
Although the GDG was presented with a high number of RCTs for this review question, there was uncertainty about the above factors, which varied in the included studies.
Key uncertainties
The economic evidence presented to the GDG on medication review was limited to review by hospital and community pharmacists. The GDG found that the evidence was conflicting and of varying quality. The GDG was uncertain about the cost effectiveness of medication reviews carried out by multidisciplinary teams or professional-led by health professionals other than hospital or community pharmacists, compared to usual care or other interventions. No economic evidence was found for primary care pharmacists carrying out medication reviews. The GDG agreed that there was uncertainty about this and that the research recommendation should include primary care pharmacists.
These uncertainties can be answered by conducting a study that will deliver good-quality evidence, such as an RCT.
Is a medication review more clinically and cost effective at reducing the suboptimal use of medicines and improving patient-reported outcomes, compared with usual care or other intervention in the UK setting?
The study should consider the cost effectiveness of the health professional(s) carrying out the medication review.
The medication review should be carried out by a multidisciplinary team or be professional-led by any health professional other than a community or hospital pharmacist to provide data to develop an economic model for cost effectiveness. There is already economic evidence available for community and hospital pharmacists (see section 8.4 ).
Research can be carried out using an RCT. Study methodology can be based on other well-conducted RCTs that have been carried out looking at medication reviews. ‘Usual care’ or other interventions would be used as a comparator. ‘Usual care’ would need to be defined in the study. A follow-up period of 1–2 years or more would capture longer-term outcomes. Outcomes for this research question should be patient-centred and include the suboptimal use of medicines, patient-reported outcomes, clinical outcomes, medicines-related problems, health and social care resource use and cost effectiveness.
- the type of medication review carried out (see section 8.1 ); the study would need to outline a framework of the medication review to help guidance developers to see the process used; they would then be better able to decide if it would affect clinical effectiveness of the intervention
- type of health professional carrying out the medication review
The GDG recognised that the key focus of the medicines optimisation agenda is to make care person-centred and to have services that support people in the optimal use of their medicines. Medication reviews can be offered to people by different health professionals at different levels, working in different settings. Resources (for example, staff and time) needed to enable routine medication review may vary locally depending on the setting and health professional availability.
Research into this area will provide guidance to organisations who may want to, or already provide, medication reviews as part of their care and enable better use of resources (for example, health professional cost and time and health and social care resources) and facilitate service delivery. This information would be useful to commissioners who may consider whether or not to commission medication reviews by providers.
All rights reserved. This material may be freely reproduced for educational and not-for-profit purposes. No reproduction by or for commercial organisations, or for commercial purposes, is allowed without the express written permission of NICE.
- Cite this Page NICE Medicines and Prescribing Centre (UK). Medicines Optimisation: The Safe and Effective Use of Medicines to Enable the Best Possible Outcomes. Manchester: National Institute for Health and Care Excellence (NICE); 2015 Mar. (NICE Guideline, No. 5.) 8, Medication review.
- PDF version of this title (3.8M)
In this Page
- Introduction
- Review question
- Evidence review
- Health economic evidence
- Evidence statements
- Evidence to recommendations
- Recommendations and research recommendations
Other titles in this collection
- National Institute for Health and Care Excellence: Guidelines
Recent Activity
- Medication review - Medicines Optimisation Medication review - Medicines Optimisation
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Interventions to optimize medication use in nursing homes: a narrative review
Anne spinewine, perrine evrard, carmel hughes.
- Author information
- Article notes
- Copyright and License information
Corresponding author.
Received 2020 Oct 20; Accepted 2021 Feb 25; Issue date 2021.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Key summary points
This review aimed to identify, describe and discuss different interventions targeting medication use optimization in nursing homes and to identify research gaps.
Prescription of the whole medication regimen or of specific medication classes was the most studied aspect. Medication review and multidisciplinary approaches appeared to be effective strategies in reducing appropriate use, but further large-scale randomized trials are needed.
Efforts to optimize medication use among nursing home residents are still needed and should focus on less evaluated intervention components, specific medication classes and medication use aspects not related to prescribing.
Keywords: Nursing homes, Older adults, Medication optimization, Potentially inappropriate prescriptions, Interventions, Implementation
Polypharmacy, medication errors and adverse drug events are frequent among nursing home residents. Errors can occur at any step of the medication use process. We aimed to review interventions aiming at optimization of any step of medication use in nursing homes.
We narratively reviewed quantitative as well as qualitative studies, observational and experimental studies that described interventions, their effects as well as barriers and enablers to implementation. We prioritized recent studies with relevant findings for the European setting.
Many interventions led to improvements in medication use. However, because of outcome heterogeneity, comparison between interventions was difficult. Prescribing was the most studied aspect of medication use. At the micro-level, medication review, multidisciplinary work, and more recently, patient-centered care components dominated. At the macro-level, guidelines and legislation, mainly for specific medication classes (e.g., antipsychotics) were employed. Utilization of technology also helped improve medication administration. Several barriers and enablers were reported, at individual, organizational, and system levels.
Overall, existing interventions are effective in optimizing medication use. However there is a need for further European well-designed and large-scale evaluations of under-researched intervention components (e.g., health information technology, patient-centered approaches), specific medication classes (e.g., antithrombotic agents), and interventions targeting medication use aspects other than prescribing (e.g., monitoring). Further development and uptake of core outcome sets is required. Finally, qualitative studies on barriers and enablers for intervention implementation would enable theory-driven intervention design.
Introduction
Medication use among nursing home residents (NHRs) is very common. Indeed, in nursing homes (NHs), polypharmacy is highly prevalent, with 91%, 74% and 65% of NHRs taking more than five, nine and 10 medications, respectively [ 1 ]. These rates of polypharmacy are higher than what has been reported in home-dwelling older adults (27.0–59.0% taking 5 or more medications [ 1 ]). Factors associated with polypharmacy among NHRs include age, cognitive status, number of prescribers, dependency and length of stay in the NH [ 1 ].
Polypharmacy, together with other factors such as altered pharmacokinetics and pharmacodynamics, and complexity of the medication use process, makes the safe use of medications for NHRs highly challenging [ 2 ]. Reported rates of adverse drug events (ADEs) in NHs range from 1.89 to 10.8 per 100 resident-months [ 3 ]. Medication errors (MEs) are common, involving 16–27% of NHRs in studies evaluating all types of MEs and 13–31% of NHRs in studies evaluating MEs occurring at transfer from and to other settings of care [ 4 ]. MEs can occur at any step of the medication use process. These steps include: prescribing, purchase and ordering, delivery, storage, preparation and administration, monitoring and medication reconciliation at transfer [ 5 ]. The minimum practices that are required to deliver high-quality care at each step have been identified and constitute opportunities for evaluation of performance [ 5 ]. The literature suggests that the majority of errors occur at the prescribing, monitoring, administration, and medication reconciliation steps [ 4 ]. In a recent review, five categories of factors related to the work system were found to affect medication safety in NHs: persons (resident and staff, e.g., number of medications, staff medication knowledge), organization (e.g., inter-professional collaboration, staff/resident ratio), tools and technology (e.g., bar-code medication system), tasks (e.g., workload and time pressure), and environment (e.g., staff interruption) [ 3 ]. It is expected that interventions to optimize medication use in NHs would address these steps and factors as priorities.
The prescribing component is an important aspect of medication optimization, as prevalence of potentially inappropriate prescriptions (PIPs) is high, and as PIP and polypharmacy have been associated with adverse outcomes such as lower quality of life, hospitalizations, falls, and frailty [ 1 , 6 – 8 ]. PIPs encompass underprescribing (failure to prescribe a needed drug), overprescribing (prescribing more drugs than needed) and misprescribing (incorrect prescribing of a needed drug) [ 2 ]. The estimated prevalence of PIPs among NHRs is 43.2% [ 9 ]. This prevalence tends to rise over time and the situation is more concerning in Europe, with higher reported point prevalence (49.0%) than these reported in North America (26.8%) or other countries (29.8%) [ 9 ]. Several factors were found to be associated with PIPs such as total number of medications taken, age, location of the NH (including country, urban versus rural), dementia and comorbidity burden [ 9 , 10 ]. The most commonly reported inappropriate medications include psychotropic drugs, medications with anticholinergic properties, antimicrobials, nonsteroidal anti-inflammatory drugs and proton-pump inhibitors [ 9 , 11 , 12 ].
Interventions to optimize medication use can be implemented at different levels of the health care system. Throughout the literature there is inconsistency in the number and definitions of these levels [ 13 ]. For this review, we distinguish between two levels. First, the micro-level refers to interventions implemented at the NH level and directed at NHRs, health care providers (HCPs) and organization of the NH itself. Second, the macro-level (also called system-level) encompasses strategies that are external to NHs but impact on their practice. These are typically but not exclusively defined at a national or regional level.
The main objective of this review is to identify, describe and discuss interventions aimed at optimization of any step of medication use in NH, in terms of content, effects, as well as barriers and enablers to their implementation. As a second objective, we aimed to identify perspectives for the future at the research and practice levels.
This review was conducted using a narrative process. We focused on interventions targeting the medication use of residents living in NHs. Relevant references were identified and selected from a search in PubMed, the authors’ existing knowledge of literature, and recent publications in geriatrics journals. Finally, we retrieved additional studies by hand-searching reference lists of identified articles. Searching additional databases (e.g., Embase, CINAHL) would have been valuable and relevant in the context of a systematic review, but this was beyond the scope of the present work.
We selected quantitative as well as qualitative studies, observational and experimental studies that described interventions, their effect as well as barriers and enablers. We only included peer-reviewed research published in English. Given the large volume of literature, we prioritized results from the most recent (systematic) reviews and original studies published after these reviews were completed. We did not restrict the country where research took place, but gave preference to studies conducted in Europe or with relevant data or messages for European settings, as judged by the research team. We did not include papers focusing on medication optimization at end of life or during palliative care which was considered beyond the scope of this review. The search strategy and papers’ selection process are presented in Fig. 1 .
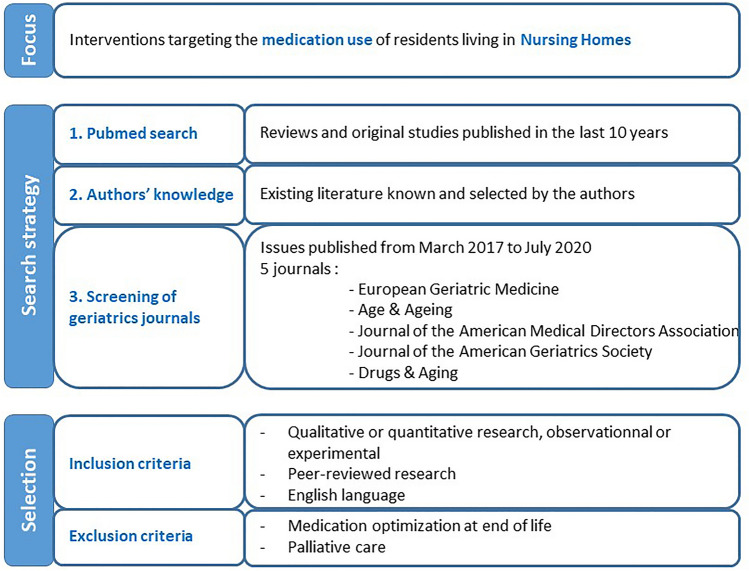
Search strategy and papers’ selection process
Because an important part of the literature focuses on the prescribing component, we first review this aspect, followed by approaches to improve other aspects of medication use. In the section on prescribing, we review separately the approaches concerned with optimizing the whole medication regimen and those concentrating on specific drugs or classes, because the approaches, their effect, as well as barriers and enablers may differ, and hence, merit separate consideration.
Interventions to optimize prescribing for the whole medication regimen
Three recent systematic reviews (SRs) evaluated the effect of micro-level interventions—largely based on medication review (MR)—to optimize prescribing in the NH setting and reported positive results on quality of prescribing [ 14 – 16 ]. A Cochrane SR highlighted four different approaches for optimization: MR, multidisciplinary case-conferencing, education for HCPs, and use of clinical decision support system (CDSS) [ 14 ]. These were used either alone, or in combination. Overall, the interventions led to identification and resolution of drug-related problems, but there was no consistent effect on resident-related outcomes [ 14 ]. In a second SR focusing on MR and including experimental and observational study designs, interventions were associated with a reduction in prescribed medications, inappropriate medications and adverse outcomes (including deaths and hospitalizations) [ 15 ]. However, high-quality cluster-randomized controlled trials evaluating CDSS effects or evaluating the impact of multidisciplinary interventions on well-defined important resident-related outcomes were lacking [ 14 , 15 ]. In terms of deprescribing, a SR of specific interventions reported a reduction of 59% of NHRs receiving at least one PIP [ 16 ]. Only interventions including a MR were associated with a reduction in all-cause mortality and number of fallers [ 16 ].
Five trials performed in European Union (EU) countries NHs were published after these SRs and are summarized in Table 1 [ 11 , 17 – 20 ]. These were all multicenter studies—three were cluster-randomized controlled trials—and involved multidisciplinary interventions mainly consisting of education of HCPs and MR. None involved a CDSS component. Participation of NHR was one component of the intervention in two studies. The study by Wouters et al. involved NHRs through a questionnaire on their preferences and experiences as a step of MR [ 18 ]. In the COSMOS study, NHRs were asked about their interest in participating in different activities [ 20 ]. Overall, results from these five trials were consistent with those of previous SRs, with positive effects on polypharmacy and PIPs—although the measures used to define PIPs varied widely across studies, and none of the tools used were specific to the NH setting. Clinical and humanistic outcomes were inconsistently evaluated (Table 2 ). Two trials reported no effect of the intervention on clinical outcomes and/or quality of life [ 11 , 18 ]. In the COSMOS study, an initial decline in quality-of-life was found in the intervention group—initial NHR unhappiness with the MR is one of the possible explanations raised by the authors—but this decrease reversed significantly during follow-up [ 20 ].
Trials conducted in European nursing homes in the last 5 years and reporting the effect of an intervention on prescribing the whole medication regimen (chronological order)
ADL activities of daily living, ARR adjusted relative risk, BAS before–after study, CG control group, CGIC clinical global impressions of change, CI confidence interval, CT controlled trial, DRP drug-related problems, GP general practitioner, IG intervention group, MR medication review, mo months, N nurse, NH nursing home, NHR nursing home resident, OR odds ratio, PIP potentially inappropriate prescription, QoL quality of life, RCT randomized controlled trial, cRCT cluster-RCT, START/STOPP screening tool to alert doctors to right treatment/screening tool of older persons’ prescriptions
Outcomes and process measures reported in trials conducted in European nursing homes in the last 5 years
ADL activities of daily living, AGS American Geriatrics Society, APID appropriate psychotropic drug use in dementia index, CGIC Clinical Global Impression of Change, DBI Drug Burden Index, DQI dementia quality of life instrument, ED emergency department, EQ-5D-3L EQ-VAS European quality of life visual analog scale, HCPs health care providers, QoL-AD quality of life in Alzheimer’s disease scale, QUALID quality of life late-stage dementia scale, QUALIDEM quality of life dementia scale, MMSE mini-mental state examination, PIP potentially inappropriate prescription, SIB-S severe impairment battery-short, START/STOPP screening tool to alert doctors to right treatment/screening tool of older persons’ prescriptions. ‘X’ without further detail means that the outcome was reported, but no details on measurement were given.
Beyond the evaluation of the effect of interventions, a clear understanding of the enablers and barriers to implementation and success is crucial for the development of future interventions. It is encouraging to see that three of the trials presented in Table 1 addressed this question, mainly through questionnaires and interviews of HCPs [ 21 – 23 ]. Wouters et al. also interviewed NHRs [ 21 ]. Overall, the interdisciplinary approaches were recognized as key elements for the success of interventions, despite organizational and time constraints. The attitude, role and competency of HCPs (physicians, pharmacists and nurses) were identified both as barriers and enablers. The need for funding MRs at the macro-level was also reported. Assessing the patient perspective was reported to be a delicate balance between the value and the barriers to a proper assessment of the patient perspective. Other qualitative studies assessed the specific barriers and enablers of deprescribing in the NH setting [ 24 , 25 ]. While many were similar to what was reported for intervention implementation, HCPs’ concerns about deprescribing and perceived reluctance of NHRs to change were more specific to deprescribing interventions. This highlights the need for deprescribing guidance and shared decision-making [ 24 , 25 ].
Interventions to optimize prescribing for specific drug classes
In the section below, we focus on three medication classes for which inappropriate use is highly prevalent and is a threat to patient safety. For each of these, we first briefly describe data on their (inappropriate) use, then review the evidence on approaches for optimization, as well as barriers and enablers for improvement. Table 3 describes five recent studies conducted in NHs in Europe, four on psychotropic drugs and one on anti-infective drugs. We found no recent EU study focusing on DAP.
Trials conducted in European nursing homes in the last 5 years and reporting the effect of an intervention on prescribing a specific medication class (chronological order)
APID appropriate psychotropic drug use in dementia, ARR adjusted relative risk, BAS before–after study, CG control group, CGIC clinical global impressions of change, CI confidence interval, CT controlled trial, DDD defined daily dose, DRP drug-related problems, GP general practitioner, IG intervention group, MR medication review, mo months, N nurse, NH nursing Home, NHR nursing home resident, OR odds ratio, P pharmacist, PIP potentially inappropriate prescription, PSM propensity score-matched, QoL quality of life, RCT randomized controlled trial, cRCT cluster-RCT, START/STOPP screening tool to alert doctors to right treatment/screening tool of older persons’ prescriptions
Psychotropic drugs
Psychotropic drugs are used extensively in NHs, with wide variation in rates of prescribing between countries. In NHs in Western European countries, antipsychotic use ranges from 12 to 59% of NHRs and antidepressant use is even higher, from 19 to 68% [ 31 ]. The use of benzodiazepine receptor agonists (BZRA, namely benzodiazepines and Z-drugs) ranges from 14.6% (Canada, [ 32 ]) to 54.4% (France, [ 33 ]). Concomitant use of several psychotropic drugs is also high with 31.5% of NHRs taking two or more such medications [ 29 ].
Beyond this high prevalence of use, frequent inappropriate use is a concern. Indeed, psychotropic drugs are often the most commonly reported inappropriate medications among NHRs [ 9 , 11 , 34 ]. The inappropriate (and off-label) use of antipsychotics for behavioral and psychological symptoms of dementia has received the most attention. This has led to national and international calls and programs for deprescribing of antipsychotics in NHs. Even though the appropriateness of antidepressants and BZRA use has been less widely studied, recent data suggest that these medicines should also give rise to concern. In a study with 2651 French NHRs receiving an antidepressant, PIP (with regard to indication, drug class, duplication and monitoring) was found in 38.4% of NHRs [ 35 ]. In a Belgian study with 418 NHRs taking a BZRA, 98% of NHRs received the BZRA for more than 4 weeks, and drug–disease and drug–drug interactions were found in two-thirds of users overall [ 36 ]. In both studies, dementia was associated with less PIP.
Data on the factors associated with (inappropriate) psychotropic drug use suggest that approaches for improvement can be considered both at the macro- and micro-levels. A recent SR found that organizational capacity, individual professional capacity, attitudes, communication and collaboration and regulation or guidelines influenced antipsychotic prescribing [ 37 ]. Similarly, factors associated with psychotropic drugs use included: staffing level or education, teamwork and communication between both on-site and visiting staff, and managerial expectations [ 13 ].
At the macro-level, a recent scoping review including 36 studies (of which only three were performed in Europe) found that mandatory strategies such as legislation (e.g., change in reimbursement, initiation of public reporting of antipsychotic use) had greater evidence of impact on drug utilization than non-mandatory macro-level strategies such as guidelines and recommendations [ 13 ]. The OBRA-87 legislation in the US led to the greatest reduction in psychotropic drug use. However, inappropriate use remains a significant issue and few studies have examined both sustainability of system-level strategies and cost-related outcomes [ 13 ].
At the micro-level, a recent narrative review of approaches for deprescribing psychotropic medications in NHRs with dementia reported that interventions should have more than one component, include multidisciplinary teams and HCPs’ training, and be person-centered [ 38 ]. The same intervention components were highlighted in a SR of factors influencing antipsychotic use among dementia NHRs [ 37 ] and in a review of interventions targeting BZRA deprescribing [ 39 ]. In Europe, a few interventions were recently evaluated, with encouraging results (Table 3 [ 26 – 29 ]). Similar to approaches targeting the whole medication regimen, training of HCPs and MR were important components of evaluated strategies. However, some more specific strategies were also tested. Patient-centered interventions were implemented in three studies. In Belgium, a quality improvement study with transition to person-centered care (e.g., through the implementation of meaningful activities for NHRs) showed a reduction in both long-term use and concomitant use of psychotropics [ 29 ]. In a Spanish study with NHRs with dementia, application of STOPP/START criteria and use of decision aids for NHRs had positive and similar effects on reducing daily dosages of psychotropic drugs, even though decision aids were less often used than STOPP/START [ 28 ]. Richter et al. investigated a person-centered care approach, which had been successfully evaluated in NHs in the UK, and adapted it to the German context. However, the program did not lead to a reduction in antipsychotic prescriptions. Reasons for differences between the UK and Germany were unclear, but the culture of care as reflected in the attitudes and beliefs of nursing staff and a lack of cooperation with physicians may have accounted for the findings [ 27 ].
Drugs with anticholinergic properties (DAP)
DAPs are associated with a wide range of peripheral and central adverse effects (e.g., delirium, fall, urinary retention), and there have been numerous calls to reduce their use [ 40 ]. A recent population-based study among NHRs with depression even found that clinically significant anticholinergic use was associated with a 31% increase in risk of death [ 41 ]. Despite such concerns, DAP are highly prevalent among NHRs. In a study conducted in Helsinki, in 2011, 85% of NHRs were taking at least one DAP [ 42 ]. Positive findings were reported in a study evaluating temporal trends from 2003 to 2017 (the anticholinergic burden decreased, and participants with dementia had a lower anticholinergic burden), but DAP use—especially antipsychotics and antidepressants—remained high [ 43 ]. This calls for action toward DAP use in NH.
A SR reported that (micro-level) interventions aiming at reducing anticholinergic burden in older adults (≥ 65) in different settings often reduced anticholinergic burden [ 40 ]. Pharmacists delivered the intervention in the majority of studies, and authors concluded that these HCPs may be well placed to implement a DAP reduction intervention [ 40 ]. Among the eight studies included, only one was conducted in NHs, in Norway. The intervention consisted of a pharmacist-initiated reduction of anticholinergic drug scale score after multidisciplinary MR. Anticholinergic drug scale scores were significantly reduced in the intervention group and remained unchanged in the control group. However, no improvement in NHRs’ cognitive function at 8 weeks was observed [ 44 ]. In another recent study conducted in New Zealand NHs, pharmacists performed deprescribing recommendations for both anticholinergic and sedative drugs. This showed that deprescribing was feasible, with 72% of recommendations implemented by physicians, without deterioration in quality of life, and with an improvement in depression and frailty scores [ 45 ]. No macro-level approaches specifically targeting DAP use were found.
These data are encouraging but remain very limited, which calls for further well-conducted, large-scale, controlled studies. The variety and heterogeneity of tools to measure and quantify anticholinergic burden remains an issue, as there is no consensus as to which of the tools is most useful in research or clinical settings [ 42 ].
Anti-infective drugs
Antimicrobials are commonly prescribed in NHs and their use is associated with antimicrobial resistance and Clostridium difficile infections. The 2016–2017 point prevalence survey performed in NHs in 24 EU countries found a crude prevalence of NHRs receiving at least one antimicrobial agent of 4.9%, with large variations across and between countries (from 0.7% in Lithuania to 10.5% in Spain and Denmark) [ 46 ]. Prophylaxis for urinary-tract infection was a frequent—and potentially inappropriate [ 47 ]—indication for antimicrobial use (representing almost one third of prescriptions) and did not significantly decline following previous surveys [ 46 ]. Inappropriate prophylactic use of antimicrobials was therefore recommended as a specific target for future interventions. Appropriate prescribing of antimicrobials in NH is challenging and influenced by several factors, such as variations in knowledge and practice among HCPs, social factors, antimicrobial resistance and the specific context of NH care (including restricted access to doctors and diagnostic tests) [ 12 ].
Antibiotic stewardship programs (ASPs) are coordinated interventions promoting the appropriate use of antibiotics to improve patients’ outcomes and reduce microbial resistance [ 48 ], which can be implemented at both the macro- and micro-levels. At the macro-level, ASPs have been mandated in American NHs since November 2017. In Europe, data on ASP indicate that there has been no increase in ASP implementation over time, and improvements in antimicrobial stewardship are urgently needed in EU NHs [ 46 ].
Recent SRs on ASP in the NH setting reported that the most commonly implemented strategies were educational materials, educational meetings, and guideline implementation, combined in multifaceted interventions [ 49 ]. Results suggested an effect on intermediate health outcomes, such as antibiotic consumption or adherence to antibiotic guidelines. However, an effect on key health outcomes such as mortality rates, hospitalizations, or Clostridium difficile infection rates was not demonstrated [ 48 – 50 ]. Moreover, the specific benefit of intervention components is unclear. In Switzerland, ASP activities including local multidisciplinary networks (micro-level strategy) and guidelines publication (macro-level strategy) led to a 22% reduction in antibacterial use over a 6-year period (Table 2 ) [ 30 ]. A recent paper described the ASP implementation experience in four European countries (Norway, The Netherlands, Poland and Sweden) where various regional or national ASP initiatives have recently been introduced [ 51 ]. The ASP components included national surveillance systems, NH-specific prescribing guidelines and national networks of healthcare institutions. No data were provided to document the effect of these initiatives on antimicrobials consumption. Future ASP implementation will need to account of enablers (e.g., the presence of study leaders, skills training for doctors and nurses, and good inter-professional communication) and barriers (e.g., pressures from residents and families, NH staff’s knowledge and belief) in order to be successful, in addition to outcome data [ 12 , 52 ].
Interventions to optimize medication reconciliation at transfer
The transition of NHRs from one setting to another increases the risk for MEs. Indeed, preventable ADEs at transition points account for 46–56% of all MEs [ 53 ] and MEs have been identified as a major source of morbidity and mortality in transitional care [ 54 ]. A possible explanation is poor communication between settings, potentially leading to prescribing errors. When questioned on ways to improve quality and safety of care transfer, NH and emergency department staff raised several strategies, including the use of a standardized transfer form, a checklist and verbal communication between settings [ 55 ].
In practice, some of these interventions have been studied at micro-level. Results from a SR on interventions to improve transitional care between NH and hospitals show that the development of a standardized unique transfer document may assist with the communication of medication lists, and that pharmacist-led review of medication lists may help identify omitted or indicated medications on transfer [ 54 ]. This is supported by results from another SR evaluating medication reconciliation interventions during NHRs’ transfer from and to the NH [ 53 ]. In most studies, a clinical pharmacist performing MR was part of the intervention. All interventions led to outcome improvement, but no study showed strong evidence in reducing medication discrepancies [ 53 ].
Existing data also suggest that HCPs believe that initiatives should be taken at the macro-level, to standardize processes during transitions. National guidance and toolkits relative to medication reconciliation in the NH setting exist in some countries such as Canada [ 56 ], but to the best of our knowledge, the impact of these initiatives on quality and safety of medication use in NHs has not been evaluated.
Interventions to optimize the preparation and administration
The preparation and administration of prescribed drugs often falls to nurses (and sometimes pharmacists for the preparation stage)—and not to NHRs themselves. Medication administration errors (MAEs) encompass different types of errors such as wrong-time errors, wrong-dose errors, omitted doses, wrong-patient errors. As an example, 27% of calls to the Quebec Poison Center for patients aged over 65 resulted from drug administration to the wrong NHR [ 57 ]. The medication administration process is prone to interruptions, and this may increase the risk of MAE. It has been reported that nurses are interrupted at a rate ranging from 0.4 to 14 times an hour [ 58 ]. Swallowing difficulties may also trigger MAEs. Indeed, it is common for nurses to modify medication dosage forms through crushing tablets or opening capsules, in order to administer a medication to NHRs with swallowing difficulties [ 59 ]. Nurses reported that this practice is challenging and would need appropriate guidelines and training [ 59 ].
To reduce the risk of MAEs and resulting harms, different approaches have been taken, and the main focus has been the implementation of technological solutions, such as electronic medication administration record (eMAR) and bar-code medication administration [ 58 , 60 – 62 ]. These technologies might be time-saving, decrease the probability of MAEs such as omitted doses and increase nurse satisfaction [ 61 , 62 ]. However, a SR on eMAR in long-term care facilities reported that eMAR implementation is low, partly because of cost barriers, and there is a lack of rigorously designed research to inform administrators and clinicians about the effect of eMARs and bar-code medication administration on MEs [ 60 ]. The use of multi-compartment compliance aids is another possible approach to reduce preparation and administration errors. A recent study in London reported that MAE rate was higher with original medication packaging than with multi-compartment compliance aids (risk ratio = 3.9, 95% CI 2.4–6.1) [ 63 ]. Limitations to their use included reduced staff alertness during administration and difficulties in identifying medication [ 63 ].
This review has highlighted that many interventions focusing on the key steps in medicine optimization led to improvement in medication use. However, some components have not been comprehensively evaluated or not in powerful designs such as randomized controlled trials. In much of the literature reviewed, there was an under-representation of aspects of medication use not related to prescribing (including monitoring). This is perhaps not surprising due to the predominance of the prescribing process in healthcare, but other aspects of medication use do require further consideration. Many studies that did focus on prescribing had common intervention components. At the micro-level MR, multidisciplinary work, and more recently, patient-centered care components dominated; at the macro-, guidelines and legislation, mainly for specific medication classes, e.g., antipsychotics, were employed. Improving administration was achieved through utilization of technology.
What was also apparent in the studies examined was the marked heterogeneity in outcome reporting and measurement across studies (Table 2 ). This makes synthesis of findings difficult and highlights the need for a more common approach across studies examining similar research questions. This may be realized through the development and use of core outcome sets (COSs). Two relevant COSs exist, for trials aimed at optimizing prescribing among NHR [ 64 ] and for trials of MR in multi-morbid older patients with polypharmacy [ 65 ]. Several outcomes of these COSs have been under-evaluated (i.e., what to measure), such as pain relief, all-cause mortality, falls, quality of life, hospital admissions and emergency visits to hospital. These are clearly important outcomes for this particular population and for the health systems. It is important that future trials refer to and use a COS. Furthermore, approaches to measurement of outcomes (i.e., how to measure) were also highly variable. PIP was measured in most studies, but a wide range of tools was used. Although many were targeted at older adults, such tools may not be appropriate for NHRs who have a higher degree of frailty. The use of tools that have been specifically developed for those who are frail [ 47 ] or living in residential care (stoppNH [ 66 ]) may be a better option.
System level (macro-level) approaches were implemented in US and Australia, but much less so in Europe. Positive effects were seen with mandatory/legislative initiatives, and it could be argued that these should be considered at the European level. However, there has been a tradition of different countries tackling approaches in nursing home care in different ways which may be a function of different cultural and political contexts [ 67 ]. Many of the concerns around prescribing of key medicines such as antipsychotics and anti-infectives are universal, and a more comprehensive, cross-country approach may be warranted.
At the micro-level, the importance of patient-centered interventions was increasingly recognized. Patient involvement or participation in the interventions was identified in two recent EU studies focusing on psychotropic drugs [ 28 , 29 ], and in one of the studies to improve prescribing for the whole medication regimen [ 18 ]. However, more research on how best to involve NHRs, and NHRs with dementia in particular, is required. In some countries, patient and public involvement is increasingly expected as part of applications for research funding [ 68 ]. A recent study introduced weekly participatory action research sessions. During these, NHRs could discuss NH initiatives and suggest improvement. Results reported a positive NHR experience and an improved quality of life [ 69 ]. However, this is a challenging area as many NHRs will have varying levels of cognitive impairment, which may limit the level of their participation.
This paper focused on a number of specific medication classes which, historically, have been viewed as problematic in this population. With regard to psychotropics, a particular focus has been on reducing the use of antipsychotic drugs, but there was little exploration of any compensatory increases in the use of other sedating psychotropic drugs [ 70 ] or in the use of non-pharmacological approaches. Measurement of clinical and humanistic outcomes was limited and heterogeneous [ 27 ], therefore, a COS for interventions targeting psychotropic/antipsychotic drug use in NHs would be welcome. Indeed, this was also seen with studies focusing on DAPs, with a plethora of scales available, but little overlap to facilitate comparison. Anti-infectives have also been extensively studied in the NH environment. There has been no concerted attempt to introduce macro-level interventions focusing on ASP, which may reflect differing prescribing practices and cultures [ 71 ], but there have been efforts to begin to standardize the important outcomes for ASP interventions [ 72 ].
We selected the medication classes above because of a legacy of concern over inappropriate use. However, other medication classes also deserve specific focus, but have been ignored. Pain control is one of the outcomes of a COS of MR in older people [ 65 ]. Inappropriate prescribing of analgesics, and opioids in particular has been described in NHRs [ 73 – 75 ]. Second, there has been little work focusing on optimizing the use of antithrombotic agents among NHRs. This is an important research gap, as bleeding and thrombotic events are the most frequent ADEs [ 4 ]. Third, data on the deprescribing of medications used for cardiovascular prevention (e.g., statins, aspirin) and for diabetes would also be welcome, as no or very limited data are available [ 76 – 78 ]. Finally, the use of proton-pump inhibitors (PPIs) is highly prevalent and often inappropriate [ 79 , 80 ]. While factors associated with both PPIs use and discontinuation have been described [ 79 , 81 ], we found only one single-center intervention study targeting PPIs deprescribing [ 82 ]. The implementation of a deprescribing guideline was not associated with a statistically significant decrease in PPIs use [ 82 ].
Health information technology (HIT) has the potential to improve medication use in this environment, specifically to reduce the occurrence of medication errors. HIT includes systems such as eMARs, electronic medication management systems, CDSS, electronic health records [ 62 ]. Long-term care facilities have lagged behind other sectors in the adoption of HIT because of the lack of funding [ 62 ]. The eMAR system was one of the most common types of technology implemented. However, this type of technological support did not extend to supporting clinical decision-making. There was little data on the effect of CDSS in NHs, but there is ongoing research on this topic ( 83 ). Its impact in the long-term environment remains to be seen as recent trials on CDSS to optimize prescribing in primary and acute care have shown negative results on clinical outcomes [ 84 , 85 ]. The relevance of alerts and usability seem to be limiting features, and these finding would be important if this technology were implemented in NHs. Other aspects of technological interventions are also lacking a strong evidence base such as the completeness and accuracy of transfer of medication information at transition moments, and the role of telemedicine.
Evidence is lacking regarding the transferability of interventions across countries and across NHs because barriers and enablers differ. Sometimes, culture and context will overwhelm any attempt to implement an approach that has worked else. However, increasingly, more attention is being paid to how interventions are developed by using recognized frameworks such as the Medical Research Council guidance on the complex interventions [ 86 ]. This systematic approach advocates for reference to existing evidence, the use of theory, modeling, pilot/feasibility testing, and implementation. There are now many more examples of interventions being developed using this approach, with a particular emphasis on theories of behavior change [ 87 ], and how barriers and enablers can be recognized [ 88 ]. A large trial evaluating the effectiveness and cost-effectiveness of a pharmacist-independent prescribing service in NHs compared to usual general practitioner-led care has been conducted in the UK and is due to report soon [ 89 ]. This trial also has an embedded process evaluation, to try to understand the mechanisms of action associated with the interventions and to explain findings in terms of fidelity to intervention performance [ 89 ]. This rigorous approach to design and evaluation enhances confidence in the conduct and findings of such studies and should be adopted by others seeking to develop and assess interventions in NHs.
The NH setting and its residents have been a focus for a range of interventions targeting the spectrum of optimizing medicines use. This review has highlighted that a number of interventions are effective, but there is a need for further well-designed and large-scale evaluations of intervention components (e.g., health information technology, patient-centered approaches), specific medication classes (e.g., antithrombotic agents) which have been less commonly studied. Interventions targeting medication use aspects other than prescribing (e.g., monitoring) should also be evaluated. Building the evidence base for effective interventions would benefit from the development and uptake of COSs to allow for synthesis of findings. Finally, qualitative studies on barriers and enablers for intervention implementation would enable theory-driven intervention design. This is likely to lead to more robust and rigorous assessments of what is effective in a patient population that has unique health care needs and challenges.
Acknowledgements
The authors thank Lorene Zerah for her critical review of the manuscript.
Author contributions
All authors have made substantial contributions to the conception of the review, analysis and interpretation of the data, and writing of the manuscript. Perrine Evrard and Anne Spinewine performed the literature search, selection of relevant articles, and initial synthesis of the data. Perrine Evrard wrote the first draft of the manuscript, and this was then critically reviewed and amended by all co-authors.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Compliance with ethical standards
Conflict of interest.
The authors declare that they have no conflicts of interest.
Ethics approval
As this is a narrative review, no ethical approval was required.
Informed consent
For this type of study, informed consent is not required.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
- 1. Jokanovic N, Tan EC, Dooley MJ, Kirkpatrick CM, Bell JS. Prevalence and factors associated with polypharmacy in long-term care facilities: a systematic review. J Am Med Dir Assoc. 2015;16(6):535.e1–12. doi: 10.1016/j.jamda.2015.03.003. [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Spinewine A, Schmader KE, Barber N, Hughes C, Lapane KL, Swine C, et al. Appropriate prescribing in elderly people: how well can it be measured and optimised? Lancet (Lond, Engl) 2007;370(9582):173–184. doi: 10.1016/S0140-6736(07)61091-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Al-Jumaili AA, Doucette WR. Comprehensive literature review of factors influencing medication safety in nursing homes: using a systems model. J Am Med Dir Assoc. 2017;18(6):470–488. doi: 10.1016/j.jamda.2016.12.069. [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Ferrah N, Lovell JJ, Ibrahim JE. Systematic review of the prevalence of medication errors resulting in hospitalization and death of nursing home residents. J Am Geriatr Soc. 2017;65(2):433–442. doi: 10.1111/jgs.14683. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Strauven G, Vanhaecht K, Anrys P, De Lepeleire J, Spinewine A, Foulon V. Development of a process-oriented quality improvement strategy for the medicines pathway in nursing homes using the SEIPS model. Res Soc Adm Pharm RSAP. 2020;16(3):360–376. doi: 10.1016/j.sapharm.2019.06.003. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Davies LE, Spiers G, Kingston A, Todd A, Adamson J, Hanratty B. Adverse outcomes of polypharmacy in older people: systematic review of reviews. J Am Med Dir Assoc. 2020;21(2):181–187. doi: 10.1016/j.jamda.2019.10.022. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Harrison SL, Kouladjian O’Donnell L, Bradley CE, Milte R, Dyer SM, Gnanamanickam ES, et al. Associations between the drug burden index, potentially inappropriate medications and quality of life in residential aged care. Drugs Aging. 2018;35(1):83–91. doi: 10.1007/s40266-017-0513-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Veronese N, Stubbs B, Noale M, Solmi M, Pilotto A, Vaona A, et al. Polypharmacy is associated with higher frailty risk in older people: an 8-year longitudinal cohort study. J Am Med Dir Assoc. 2017;18(7):624–628. doi: 10.1016/j.jamda.2017.02.009. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. Morin L, Laroche ML, Texier G, Johnell K. Prevalence of potentially inappropriate medication use in older adults living in nursing homes: a systematic review. J Am Med Dir Assoc. 2016;17(9):862.e1–9. doi: 10.1016/j.jamda.2016.06.011. [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Anrys PMS, Strauven GC, Foulon V, Degryse JM, Henrard S, Spinewine A. Potentially inappropriate prescribing in belgian nursing homes: prevalence and associated factors. J Am Med Dir Assoc. 2018;19(10):884–890. doi: 10.1016/j.jamda.2018.06.010. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Strauven G, Anrys P, Vandael E, Henrard S, De Lepeleire J, Spinewine A, et al. Cluster-controlled trial of an intervention to improve prescribing in nursing homes study. J Am Med Dir Assoc. 2019;20(11):1404–1411. doi: 10.1016/j.jamda.2019.06.006. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Fleming A, Bradley C, Cullinan S, Byrne S. Antibiotic prescribing in long-term care facilities: a meta-synthesis of qualitative research. Drugs Aging. 2015;32(4):295–303. doi: 10.1007/s40266-015-0252-2. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Langford AV, Chen TF, Roberts C, Schneider CR. Measuring the impact of system level strategies on psychotropic medicine use in aged care facilities: a scoping review. Res Soc Adm Pharm RSAP. 2020;16(6):746–759. doi: 10.1016/j.sapharm.2019.08.035. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Alldred DP, Kennedy MC, Hughes C, Chen TF, Miller P. Interventions to optimise prescribing for older people in care homes. Cochrane Database Syst Rev. 2016;2:Cd009095. doi: 10.1002/14651858.CD009095.pub3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Thiruchelvam K, Hasan SS, Wong PS, Kairuz T. Residential aged care medication review to improve the quality of medication use: a systematic review. J Am Med Dir Assoc. 2017;18(1):87.e1–.e14. doi: 10.1016/j.jamda.2016.10.004. [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Kua CH, Mak VSL, Huey Lee SW. Health outcomes of deprescribing interventions among older residents in nursing homes: a systematic review and meta-analysis. J Am Med Dir Assoc. 2019;20(3):362–72.e11. doi: 10.1016/j.jamda.2018.10.026. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Fog AF, Kvalvaag G, Engedal K, Straand J. Drug-related problems and changes in drug utilization after medication reviews in nursing homes in Oslo, Norway. Scand J Prim Health Care. 2017;35(4):329–335. doi: 10.1080/02813432.2017.1397246. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. Wouters H, Scheper J, Koning H, Brouwer C, Twisk JW, van der Meer H, et al. Discontinuing inappropriate medication use in nursing home residents: a cluster randomized controlled trial. Ann Intern Med. 2017;167(9):609–617. doi: 10.7326/M16-2729. [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Cool C, Cestac P, McCambridge C, Rouch L, de Souto BP, Rolland Y, et al. Reducing potentially inappropriate drug prescribing in nursing home residents: effectiveness of a geriatric intervention. Br J Clin Pharmacol. 2018;84(7):1598–1610. doi: 10.1111/bcp.13598. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. Husebø BS, Ballard C, Aarsland D, Selbaek G, Slettebo DD, Gulla C, et al. The effect of a multicomponent intervention on quality of life in residents of nursing homes: a randomized controlled trial (COSMOS) J Am Med Dir Assoc. 2019;20(3):330–339. doi: 10.1016/j.jamda.2018.11.006. [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Wouters H, Foster JM, Ensink A, O'Donnell LK, Zuidema SU, Boersma F, et al. Barriers and facilitators of conducting medication reviews in nursing home residents: a qualitative study. Front Pharmacol. 2019;10:1026. doi: 10.3389/fphar.2019.01026. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 22. Gulla C, Flo E, Kjome RLS, Husebo BS. Implementing a novel strategy for interprofessional medication review using collegial mentoring and systematic clinical evaluation in nursing homes (COSMOS) BMC Geriatr. 2019;19(1):130. doi: 10.1186/s12877-019-1139-6. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 23. Anrys P, Strauven G, Roussel S, Vande Ginste M, De Lepeleire J, Foulon V, et al. Process evaluation of a complex intervention to optimize quality of prescribing in nursing homes (COME-ON study) Implement Sci IS. 2019;14(1):104. doi: 10.1186/s13012-019-0945-8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. Turner JP, Edwards S, Stanners M, Shakib S, Bell JS. What factors are important for deprescribing in Australian long-term care facilities? Perspectives of residents and health professionals. BMJ Open. 2016;6(3):e009781. doi: 10.1136/bmjopen-2015-009781. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 25. Foley RA, Hurard LL, Cateau D, Koutaissoff D, Bugnon O, Niquille A. Physicians’, nurses’ and pharmacists’ perceptions of determinants to deprescribing in nursing homes considering three levels of action: a qualitative study. Pharm (Basel, Switz) 2020;8(1):17. doi: 10.3390/pharmacy8010017. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. van der Spek K, Koopmans R, Smalbrugge M, Nelissen-Vrancken M, Wetzels RB, Smeets CHW, et al. The effect of biannual medication reviews on the appropriateness of psychotropic drug use for neuropsychiatric symptoms in patients with dementia: a randomised controlled trial. Age Ageing. 2018;47(3):430–437. doi: 10.1093/ageing/afy001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Richter C, Berg A, Langner H, Meyer G, Köpke S, Balzer K, et al. Effect of person-centred care on antipsychotic drug use in nursing homes (EPCentCare): a cluster-randomised controlled trial. Age Ageing. 2019;48(3):419–425. doi: 10.1093/ageing/afz016. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 28. Weeks WB, Mishra MK, Curto D, Petersen CL, Cano P, Hswen Y, et al. Comparing three methods for reducing psychotropic use in older demented spanish care home residents. J Am Geriatr Soc. 2019;67(7):1444–1453. doi: 10.1111/jgs.15855. [ DOI ] [ PubMed ] [ Google Scholar ]
- 29. Wauters M, Elseviers M, Peeters L, De Meester D, Christiaens T, Petrovic M. Reducing psychotropic drug use in nursing homes in belgium: an implementation study for the roll-out of a practice improvement initiative. Drugs Aging. 2019;36(8):769–780. doi: 10.1007/s40266-019-00686-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Plüss-Suard C, Niquille A, Héquet D, Krähenbühl S, Pichon R, Zanetti G, et al. Decrease in antibacterial use and facility-level variability after the introduction of guidelines and implementation of physician-pharmacist-nurse quality circles in swiss long-term care facilities. J Am Med Dir Assoc. 2020;21(1):78–83. doi: 10.1016/j.jamda.2019.05.016. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Janus SI, van Manen JG, Izerman MJ, Zuidema SU. Psychotropic drug prescriptions in Western European nursing homes. Int Psychogeriatr. 2016;28(11):1775–1790. doi: 10.1017/S1041610216001150. [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Ivers NM, Taljaard M, Giannakeas V, Reis C, Williams E, Bronskill S. Public reporting of antipsychotic prescribing in nursing homes: population-based interrupted time series analyses. BMJ Qual Saf. 2019;28(2):121–131. doi: 10.1136/bmjqs-2018-007840. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 33. de Souto BP, Lapeyre-Mestre M, Cestac P, Vellas B, Rolland Y. Effects of a geriatric intervention aiming to improve quality care in nursing homes on benzodiazepine use and discontinuation. Br J Clin Pharmacol. 2016;81(4):759–767. doi: 10.1111/bcp.12847. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 34. Storms H, Marquet K, Aertgeerts B, Claes N. Prevalence of inappropriate medication use in residential long-term care facilities for the elderly: a systematic review. Eur J Gen Pract. 2017;23(1):69–77. doi: 10.1080/13814788.2017.1288211. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Abdeljalil A-B, Arbus C, Montastruc F, de Souto BP, André L, Vellas B, et al. Antidepressants in nursing homes for dependent older patients: the cross-sectional associations of institutional factors with prescription conformance. Eur Geriatr Med. 2019;10(3):421–430. doi: 10.1007/s41999-019-00189-x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Evrard P, Henrard S, Foulon V, Spinewine A. Benzodiazepine use and deprescribing in belgian nursing homes: results from the COME-ON study. J Am Geriatr Soc. 2020;68:2768–2777. doi: 10.1111/jgs.16751. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Walsh KA, Dennehy R, Sinnott C, Browne J, Byrne S, McSharry J, et al. Influences on decision-making regarding antipsychotic prescribing in nursing home residents with dementia: a systematic review and synthesis of qualitative evidence. J Am Med Dir Assoc. 2017;18(10):8971–9012. doi: 10.1016/j.jamda.2017.06.032. [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Harrison SL, Cations M, Jessop T, Hilmer SN, Sawan M, Brodaty H. Approaches to deprescribing psychotropic medications for changed behaviours in long-term care residents living with dementia. Drugs Aging. 2019;36(2):125–136. doi: 10.1007/s40266-018-0623-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 39. Ng BJ, Le Couteur DG, Hilmer SN. Deprescribing benzodiazepines in older patients: impact of interventions targeting physicians, pharmacists, and patients. Drugs Aging. 2018;35(6):493–521. doi: 10.1007/s40266-018-0544-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Nakham A, Myint PK, Bond CM, Newlands R, Loke YK, Cruickshank M. Interventions to reduce anticholinergic burden in adults aged 65 and older: a systematic review. J Am Med Dir Assoc. 2020;21(2):172–80.e5. doi: 10.1016/j.jamda.2019.06.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Chatterjee S, Bali V, Carnahan RM, Chen H, Johnson ML, Aparasu RR. Risk of mortality associated with anticholinergic use in elderly nursing home residents with depression. Drugs Aging. 2017;34(9):691–700. doi: 10.1007/s40266-017-0475-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Aalto UL, Finne-Soveri H, Kautiainen H, Roitto H-M, Öhman H, Pitkälä KH. Use of anticholinergic drugs according to various criteria and their association with psychological well-being and mortality in long-term care facilities. J Am Med Dir Assoc. 2019;20(9):1156–1162. doi: 10.1016/j.jamda.2019.02.007. [ DOI ] [ PubMed ] [ Google Scholar ]
- 43. Aalto UL, Roitto H-M, Finne-Soveri H, Kautiainen H, Pitkälä KH. Temporal trends in the use of anticholinergic drugs among older people living in long-term care facilities in helsinki. Drugs Aging. 2020;37(1):27–34. doi: 10.1007/s40266-019-00720-6. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 44. Kersten H, Molden E, Tolo IK, Skovlund E, Engedal K, Wyller TB. Cognitive effects of reducing anticholinergic drug burden in a frail elderly population: a randomized controlled trial. J Gerontol Ser A. 2012;68(3):271–278. doi: 10.1093/gerona/gls176. [ DOI ] [ PubMed ] [ Google Scholar ]
- 45. Ailabouni N, Mangin D, Nishtala PS. DEFEAT-polypharmacy: deprescribing anticholinergic and sedative medicines feasibility trial in residential aged care facilities. Int J Clin Pharm. 2019;41(1):167–178. doi: 10.1007/s11096-019-00784-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 46. Ricchizzi E, Latour K, Kärki T, Buttazzi R, Jans B, Moro ML, et al. Antimicrobial use in European long-term care facilities: results from the third point prevalence survey of healthcare-associated infections and antimicrobial use, 2016 to 2017. Eurosurveillance. 2018;23(46):1800394. doi: 10.2807/1560-7917.ES.2018.23.46.1800394. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 47. Lavan AH, Gallagher P, Parsons C, O'Mahony D. STOPPFrail (Screening tool of older persons prescriptions in frail adults with limited life expectancy): consensus validation. Age Ageing. 2017;46(4):600–607. doi: 10.1093/ageing/afx005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 48. Feldstein D, Sloane PD, Feltner C. Antibiotic stewardship programs in nursing homes: a systematic review. J Am Med Dir Assoc. 2018;19(2):110–116. doi: 10.1016/j.jamda.2017.06.019. [ DOI ] [ PubMed ] [ Google Scholar ]
- 49. Wu JHC, Langford BJ, Daneman N, Friedrich JO, Garber G. Antimicrobial stewardship programs in long-term care settings: a meta-analysis and systematic review. J Am Geriatr Soc. 2019;67(2):392–399. doi: 10.1111/jgs.15675. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 50. Nguyen HQ, Tunney MM, Hughes CM. Interventions to improve antimicrobial stewardship for older people in care homes: a systematic review. Drugs Aging. 2019;36(4):355–369. doi: 10.1007/s40266-019-00637-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 51. Van Buul LW, Monnier AA, Sundvall P-D, Ulleryd P, Godycki-Cwirko M, Kowalczyk A, et al. Antibiotic stewardship in European nursing homes: experiences from the Netherlands, Norway, Poland, and Sweden. J Am Med Dir Assoc. 2020;21(1):34–40.e1. doi: 10.1016/j.jamda.2019.10.005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 52. Arnold SH, Olesen JA, Jensen JN, Bjerrum L, Holm A, Kousgaard MB. Development of a tailored, complex intervention for clinical reflection and communication about suspected urinary tract infections in nursing home residents. Antibiot (Basel, Switz) 2020;9(6):30. doi: 10.3390/antibiotics9060360. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 53. Chhabra PT, Rattinger GB, Dutcher SK, Hare ME, Parsons KL, Zuckerman IH. Medication reconciliation during the transition to and from long-term care settings: a systematic review. Res Soc Adm Pharm RSAP. 2012;8(1):60–75. doi: 10.1016/j.sapharm.2010.12.002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 54. LaMantia MA, Scheunemann LP, Viera AJ, Busby-Whitehead J, Hanson LC. Interventions to improve transitional care between nursing homes and hospitals: a systematic review. J Am Geriatr Soc. 2010;58(4):777–782. doi: 10.1111/j.1532-5415.2010.02776.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 55. Terrell KM, Miller DK. Strategies to improve care transitions between nursing homes and emergency departments. J Am Med Dir Assoc. 2011;12(8):602–605. doi: 10.1016/j.jamda.2010.09.007. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 56. Scholl I, Kriston L, Dirmaier J, Buchholz A, Harter M. Development and psychometric properties of the Shared Decision Making Questionnaire–physician version (SDM-Q-Doc) Patient Educ Couns. 2012;88(2):284–290. doi: 10.1016/j.pec.2012.03.005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Dubé P-A, Portelance J, Corbeil O, Tessier M, St-Onge M. Drug administration to the wrong nursing home residents reported to the québec poison center: a retrospective study. J Am Med Dir Assoc. 2018;19(10):891–895. doi: 10.1016/j.jamda.2018.05.007. [ DOI ] [ PubMed ] [ Google Scholar ]
- 58. Odberg KR, Hansen BS, Aase K, Wangensteen S. Medication administration and interruptions in nursing homes: a qualitative observational study. J Clin Nurs. 2018;27(5–6):1113–1124. doi: 10.1111/jocn.14138. [ DOI ] [ PubMed ] [ Google Scholar ]
- 59. Forough AS, Wong SYM, Lau ETL, Santos JMS, Kyle GJ, Steadman KJ, et al. Nurse experiences of medication administration to people with swallowing difficulties living in aged care facilities: a systematic review of qualitative evidence. JBI Database Syst Rev Implement Rep. 2018;16(1):71–86. doi: 10.11124/JBISRIR-2016-003334. [ DOI ] [ PubMed ] [ Google Scholar ]
- 60. Fuller AEC, Guirguis LM, Sadowski CA, Makowsky MJ. Electronic medication administration records in long-term care facilities: a scoping review. J Am Geriatr Soc. 2018;66(7):1428–1436. doi: 10.1111/jgs.15384. [ DOI ] [ PubMed ] [ Google Scholar ]
- 61. Qian S, Yu P, Hailey D, Wang N, Bhattacherjee A. Medication administration process in a residential aged care home: an observational study. J Nurs Manag. 2018;26(8):1033–1043. doi: 10.1111/jonm.12632. [ DOI ] [ PubMed ] [ Google Scholar ]
- 62. Kruse CS, Mileski M, Syal R, MacNeil L, Chabarria E, Basch C. Evaluating the relationship between health information technology and safer-prescribing in the long-term care setting: a systematic review. Technol Health Care. 2020;28:1–14. doi: 10.3233/THC-202196. [ DOI ] [ PubMed ] [ Google Scholar ]
- 63. Gilmartin-Thomas JF, Smith F, Wolfe R, Jani Y. A comparison of medication administration errors from original medication packaging and multi-compartment compliance aids in care homes: a prospective observational study. Int J Nurs Stud. 2017;72:15–23. doi: 10.1016/j.ijnurstu.2017.03.008. [ DOI ] [ PubMed ] [ Google Scholar ]
- 64. Millar AN, Daffu-O'Reilly A, Hughes CM, Alldred DP, Barton G, Bond CM, et al. Development of a core outcome set for effectiveness trials aimed at optimising prescribing in older adults in care homes. Trials. 2017;18(1):175. doi: 10.1186/s13063-017-1915-6. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 65. Beuscart JB, Knol W, Cullinan S, Schneider C, Dalleur O, Boland B, et al. International core outcome set for clinical trials of medication review in multi-morbid older patients with polypharmacy. BMC Med. 2018;16(1):21. doi: 10.1186/s12916-018-1007-9. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 66. Khodyakov D, Ochoa A, Olivieri-Mui BL, Bouwmeester C, Zarowitz BJ, Patel M, et al. Screening tool of older person’s prescriptions/screening tools to alert doctors to right treatment medication criteria modified for US nursing home setting. J Am Geriatr Soc. 2017;65(3):586–591. doi: 10.1111/jgs.14689. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 67. Hughes CM, Roughead E, Kerse N. Improving use of medicines for older people in long-term care: contrasting the policy approach of four countries. Healthc Policy Politiques de Sante. 2008;3(3):154–167. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 68. Brownlee K, Devins G, Flanigan M, Fleming J, Morehouse R, Moscovitch A, et al. Are there gender differences in the prescribing of hypnotic medications for insomnia? England. 2003;18:69–73. doi: 10.1002/hup.452. [ DOI ] [ PubMed ] [ Google Scholar ]
- 69. Van Malderen L, De Vriendt P, Mets T, Verté D, Gorus E. Experiences and effects of structurally involving residents in the nursing home by means of participatory action research: a mixed method study. J Am Med Dir Assoc. 2017;18(6):495–502. doi: 10.1016/j.jamda.2016.12.072. [ DOI ] [ PubMed ] [ Google Scholar ]
- 70. Kales HC, Gitlin LN, Lyketsos CG. When less is more, but still not enough: why focusing on limiting antipsychotics in people with dementia is the wrong policy imperative. J Am Med Dir Assoc. 2019;20(9):1074–1079. doi: 10.1016/j.jamda.2019.05.022. [ DOI ] [ PubMed ] [ Google Scholar ]
- 71. McClean P, Hughes C, Tunney M, Goossens H, Jans B. Antimicrobial prescribing in European nursing homes. J Antimicrob Chemother. 2011;66(7):1609–1616. doi: 10.1093/jac/dkr183. [ DOI ] [ PubMed ] [ Google Scholar ]
- 72. Nguyen HQ, Bradley DT, Tunney MM, Hughes CM. Antimicrobial stewardship in care homes: outcomes of importance to stakeholders. J Hosp Infect. 2020;104(4):582–591. doi: 10.1016/j.jhin.2019.12.024. [ DOI ] [ PubMed ] [ Google Scholar ]
- 73. La Frenais FL, Bedder R, Vickerstaff V, Stone P, Sampson EL. Temporal trends in analgesic use in long-term care facilities: a systematic review of international prescribing. J Am Geriatr Soc. 2018;66(2):376–382. doi: 10.1111/jgs.15238. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 74. Hunnicutt JN, Chrysanthopoulou SA, Ulbricht CM, Hume AL, Tjia J, Lapane KL. Prevalence of long-term opioid use in long-stay nursing home residents. J Am Geriatr Soc. 2018;66(1):48–55. doi: 10.1111/jgs.15080. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 75. Könner F, Budnick A, Kuhnert R, Wulff I, Kalinowski S, Martus P, et al. Interventions to address deficits of pharmacological pain management in nursing home residents—a cluster-randomized trial. Eur J Pain (Lond, Engl) 2015;19(9):1331–1341. doi: 10.1002/ejp.663. [ DOI ] [ PubMed ] [ Google Scholar ]
- 76. Malekakan A, van Hout H, Onder G, Finne-Soveri H, van der Roest H, van Marum R. Prevalence of preventive cardiovascular medication use in nursing home residents. Room for deprescribing the SHELTER Study. J Am Med Dir Assoc. 2017;18(12):1037–1042. doi: 10.1016/j.jamda.2017.06.022. [ DOI ] [ PubMed ] [ Google Scholar ]
- 77. Goyal P, Anderson TS, Bernacki GM, Marcum ZA, Orkaby AR, Kim D, et al. Physician perspectives on deprescribing cardiovascular medications for older adults. J Am Geriatr Soc. 2020;68(1):78–86. doi: 10.1111/jgs.16157. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 78. Jokanovic N, Kautiainen H, Bell JS, Tan ECK, Pitkala KH. Change in prescribing for secondary prevention of stroke and coronary heart disease in finnish nursing homes and assisted living facilities. Drugs Aging. 2019;36(6):571–579. doi: 10.1007/s40266-019-00656-x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 79. Teramura-Grönblad M, Hosia-Randell H, Muurinen S, Pitkala K. Use of proton-pump inhibitors and their associated risks among frail elderly nursing home residents. Scand J Prim Health Care. 2010;28(3):154–159. doi: 10.3109/02813432.2010.493315. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 80. Rane PP, Guha S, Chatterjee S, Aparasu RR. Prevalence and predictors of non-evidence based proton pump inhibitor use among elderly nursing home residents in the US. Res Soc Adm Pharm RSAP. 2017;13(2):358–363. doi: 10.1016/j.sapharm.2016.02.012. [ DOI ] [ PubMed ] [ Google Scholar ]
- 81. Linsky A, Hermos JA, Lawler EV, Rudolph JL. Proton pump inhibitor discontinuation in long-term care. J Am Geriatr Soc. 2011;59(9):1658–1664. doi: 10.1111/j.1532-5415.2011.03545.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 82. Thompson W, Hogel M, Li Y, Thavorn K, O'Donnell D, McCarthy L, et al. Effect of a proton pump inhibitor deprescribing guideline on drug usage and costs in long-term care. J Am Med Dir Assoc. 2016;17(7):673.e1–4. doi: 10.1016/j.jamda.2016.04.020. [ DOI ] [ PubMed ] [ Google Scholar ]
- 83. Mestres Gonzalvo C, de Wit HA, van Oijen BP, Hurkens KP, Janknegt R, Schols JM, et al. Supporting clinical rules engine in the adjustment of medication (SCREAM): protocol of a multicentre, prospective, randomised study. BMC Geriatr. 2017;17(1):35. doi: 10.1186/s12877-017-0426-3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 84. O'Mahony D, Gudmundsson A, Soiza RL, Petrovic M, Jose Cruz-Jentoft A, Cherubini A, et al. Prevention of adverse drug reactions in hospitalized older patients with multi-morbidity and polypharmacy: the SENATOR* randomized controlled clinical trial. Age Ageing. 2020;49(4):605–614. doi: 10.1093/ageing/afaa072. [ DOI ] [ PubMed ] [ Google Scholar ]
- 85. Rieckert A, Reeves D, Altiner A, Drewelow E, Esmail A, Flamm M, et al. Use of an electronic decision support tool to reduce polypharmacy in elderly people with chronic diseases: cluster randomised controlled trial. BMJ (Clin Res ed) 2020;369:m1822. doi: 10.1136/bmj.m1822. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 86. Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ (Clin Res ed) 2008;337:a1655. doi: 10.1136/bmj.a1655. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 87. Hughes CM, Cadogan CA, Ryan CA. Development of a pharmacy practice intervention: lessons from the literature. Int J Clin Pharm. 2016;38(3):601–606. doi: 10.1007/s11096-015-0180-6. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 88. Cadogan CA, Ryan C, Hughes C. Making the case for change: what researchers need to consider when designing behavior change interventions aimed at improving medication dispensing. Res Soc Adm Pharm RSAP. 2016;12(1):149–153. doi: 10.1016/j.sapharm.2015.04.002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 89. Bond CM, Holland R, Alldred DP, Arthur A, Barton G, Birt L, et al. Protocol for the process evaluation of a cluster randomised controlled trial to determine the effectiveness and cost-effectiveness of independent pharmacist prescribing in care home: the CHIPPS study. Trials. 2020;21(1):439. doi: 10.1186/s13063-020-04264-8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (846.3 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
You are using an outdated browser
Unfortunately Ausmed.com does not support your browser. Please upgrade your browser to continue.
Conducting a Medication Review

Medicines are associated with more errors and adverse events than any other healthcare intervention, resulting in increased costs and reduced quality of care (SHPA 2015).
Medication reviews are integral in reducing the prevalence of adverse events and ensuring that medicines are being used safely and appropriately.
What is a Medication Review?
A medication review is a systematic evaluation of a patient’s medicines. It is an interprofessional process that aims to ensure medicines are being used safely and effectively at all times (CEC 2019).
During a medication review, a practitioner works together with the patient to assess the risks and benefits of all medicines being taken by the patient. This may lead to decisions being made about the patient’s medication regimen (PSA 2020).
The two main goals of a medication review are to:
- Facilitate the effective and quality use of medicines
- Reduce the likelihood of medicine-related issues such as adverse drug reactions (ADRs) .
Any issues or potential issues found during a medication review should be documented and addressed, and appropriate recommendations should be made to counter identified risks (CEC 2019).
Rather than solely being a pharmacist’s role, medication reviews are an interprofessional responsibility. Other members of the care team may conduct reviews when medicines are being prescribed, dispensed and administered, as long as they are appropriately trained to do so (ACSQHC 2023; CEC 2019). Ideally, a multidisciplinary collaborative approach is used.

Medication Reviews in the National Safety and Quality Health Service Standards
Medication reviews are outlined in Action 4.10 of the National Safety and Quality Health Service Standards , under Standard 4: Medication Safety Standard .
This action states that medication reviews should be conducted and documented in order to ensure medicine use is optimised and the risk of medicine-related problems is reduced (ACSQHC 2023).
Health service organisations are required to:
- Conduct medication reviews in accordance with evidence and best practice
- Prioritise reviews based on patients’ clinical needs
- Document reviews and any recommendations made.
(ACSQHC 2023)
Medication Reviews Under the Strengthened Aged Care Quality Standards
Standard 5: Clinical Care - Outcome 5.3: Safe and quality use of medicines under the strengthened Aged Care Quality Standards (Action 5.3.2) requires aged care providers to conduct medication reviews for an older person:
- At the start of care, during care transitions and annually for ongoing care
- When there is a change in diagnosis, a decline in behaviour, cognition, mental or physical condition, or when the person becomes acutely unwell
- When there is polypharmacy that can potentially be deprescribed
- When a new medicine is started, or there is a change to an existing medicine or the older person’s medication management plan
- When an adverse event potentially related to the older person’s medicines occurs.
(ACQSC 2024)
Medication Review v Medication Reconciliation
Keep in mind that medication reviews and medication reconciliation are different, but related processes. While medication reviews focus on optimising medicine use, identifying issues and implementing recommendations, medication reconciliation is more related to interprofessional communication and resolving discrepancies (CEC 2019).
Generally, medication reconciliation should be undertaken prior to a review, as this will ensure the patient’s medication history is as comprehensive as possible, allowing for a more accurate review (CEC 2019).
Who Requires a Medication Review?
There are a number of reasons why a medication review might be needed. The patient, their carers, medical practitioners, pharmacists or health professionals involved in the patient’s care are all able to indicate that a review may be necessary, but the medical practitioner is responsible for making the decision to refer (PSA 2020). In the community and in aged care facilities, funding is available for General practitioners and accredited pharmacists under the Medical Benefits Scheme (MBS) .
Every organisation should have risk assessment criteria for identifying patients who require a medication review. Priority should be given to patients who are at higher risk of experiencing medicine-related problems or ADRs (ACSQHC 2023).
Patients who meet any of the following risk factors are more susceptible to medicine-related problems and adverse events, and may benefit from a medication review:
- Patients over the age of 65
- Patients who have recently experienced a medication-related issue or ADR
- Patients who are non-adherent with medicines (or suspected to be)
- Patients who are taking more than 5 medicines, or more than 12 doses of medicines per day
- Patients who have not achieved or maintained goals of medication therapy
- Patients with several medicine prescribers
- Patients who have chronic conditions that are associated with a high risk of unplanned hospital admission (e.g. COPD, heart failure)
- Patients with three or more chronic conditions
- Patients who have recently been discharged from acute care
- Patients who have frequent unplanned hospital admissions
- Patients who have had an unplanned readmission within 28 days of discharge
- Patients who have recently been admitted to residential aged care
- Patients who have had significant changes to their medication regimen within the past three months
- Patients taking medicines known to be high-risk such as opioids and psychotropic medicines
- Patients with functional issues that increase the risk of harm or decrease the likelihood of benefitting from medicines (e.g. frailty, cognitive impairment)
- Patients experiencing prescription cascade (when a medicine is prescribed to manage the adverse effects of another medicine)
- Patients who are having difficulty managing devices such as inhalers or subcutaneous injections
- Patients with a BMI greater than 35
- Patients with no regular general practitioner
- Patients who are having difficulty understanding and following their medication regimen
- Culturally or linguistically diverse patients, or patients experiencing literacy difficulties.
(PSA 2020; SHPA 2015)

What Does a Medication Review Involve?
A structured medication review should comprise:
- All current medicines being taken by the patient (prescribed, over-the-counter and complementary) and what they are being used for
- All past medicine-related orders and administration records
- Anaesthetic and operative records
- Ceased medicine orders
- How safe, appropriate and effective current medicines are, and whether they are being used in accordance with guidelines
- Whether the patient has experienced any ADRs or has risk factors that would predispose them to an ADR
- Any monitoring that is required
- The patient’s views, understanding, concerns, questions and problems related to their medicines.
(ACSQHC 2023; NICE 2015)
Reviewing Medicines
Each medicine currently being taken by the patient (i.e. existing or newly prescribed) needs to be assessed for clarity , validity and appropriateness (ACSQHC 2023).
The following questions should be asked:
(CEC 2019; SHPA 2013)
With these points in mind, you should also consider:
- Whether there is documented reasoning or evidence indicating the use of medicine
- Whether the patient still needs the medicine
- Whether the medicine is working
- Whether there are any risks associated with the medicine
- Any monitoring that is required, if necessary
- Whether any patient-specific issues will affect the use of the medicine.
Working With Patients

Medication reviews should be patient-centred (WA DoH 2018), taking into account the patient’s experience with the medicines they are taking (ACSQHC 2023).
The patient should be supported to participate in the review process to the extent that they choose and provide input about the options, benefits and risks of their medicines (PSA 2020).
When conducting a medication review, the patient’s satisfaction with their medicines should be assessed to determine whether they are having a positive care experience (e.g. whether they are experiencing any medicine-related problems). If the patient has a chronic condition, their quality of life and life expectancy should be considered when making decisions (ACSQHC 2023).
Discussing when, how and whether the patient has been taking their prescribed medicines will provide a greater understanding of the patient’s experience and allow any issues to be identified and addressed (ACSQHC 2023).
Medication reviews can help ensure that medicines are always being used safely and appropriately while reducing the risk of medicine-related problems and ADRs. They are also a useful way of gauging patient experience and satisfaction so that the patient’s medicine regimen can be tailored to better suit their needs.
All reviews and recommendations that arise should be appropriately documented, and medicines should always be prescribed, dispensed and administered in consultation with the required information and patient records.
- Aged Care Quality and Safety Commission 2024, Draft Provider Guidance Standard 5 , Australian Government, viewed 15 May 2024, https://www.agedcarequality.gov.au/resource-library/draft-provider-guidance-standard-5
- Australian Commission on Safety and Quality in Health Care 2023, Action 4.10 Medication Review , Australian Government, viewed 12 October 2023, https://www.safetyandquality.gov.au/standards/national-safety-and-quality-health-service-nsqhs-standards/medication-safety-standard/continuity-medication-management/action-410
- Clinical Excellence Commission 2019, A Guide to Medication Reviews for NSW Health Services 2019 , New South Wales Government, viewed 12 October 2023, http://www.cec.health.nsw.gov.au/__data/assets/pdf_file/0016/554110/A-Guide-to-Medication-Reviews-for-NSW-Health-Services-2019.PDF
- The National Institute for Health and Care Excellence 2015, Medicines Optimisation: The Safe and Effective Use of Medicines to Enable the Best Possible Outcomes , NICE, viewed 12 October 2023, https://www.nice.org.uk/guidance/ng5/resources/medicines-optimisation-the-safe-and-effective-use-of-medicines-to-enable-the-best-possible-outcomes-pdf-51041805253
- Pharmaceutical Society of Australia 2020, Guidelines for Comprehensive Medication Management Reviews , PSA, viewed 12 October 2023, https://www.ppaonline.com.au/wp-content/uploads/2020/04/PSA-Guidelines-for-Comprehensive-Medication-Management-Reviews.pdf
- The Society of Hospital Pharmacists of Australia 2013, Compiled Quick Guides SHPA , SHPA, viewed 12 October 2023, https://shpa.org.au/publicassets/c0a132c4-de53-ec11-80dd-005056be03d0/quick_guides_e-book_2013.pdf
- The Society of Hospital Pharmacists of Australia 2015, Fact Sheet: Risk Factors for Medication-related Problems , SHPA, viewed 12 October 2023, https://shpa.org.au/publicassets/7f4d5fd6-de53-ec11-80dd-005056be03d0/shpa_fact_sheet_risk_factors_for_medication_related_problems_june_2015.pdf
- Western Australia Department of Health 2018, Best Practice Principles for Medication Review: Guidance Document , Government of Western Australia, viewed 12 October 2023, https://ww2.health.wa.gov.au/~/media/Files/Corporate/Policy%20Frameworks/Clinical%20Governance%20Safety%20and%20Quality/Policy/Medication%20Review%20Policy/Supporting/Best-Practice-Principles-for-Medication-Review.pdf
Additional Resources
- Draft Provider Guidance Standard 5 | ACQSC
- Stronger Standards, Better Aged Care Program | ACQSC
- Action 4.10: Medication Review | ACSQHC
- A Guide to Medication Reviews for NSW Health Services 2019 | CEC
- Guidelines for Comprehensive Medication Management Reviews | PSA
- Fact Sheet: Risk Factors for Medication-related Problems | SHPA
Test Your Knowledge
Question 1 of 3
True or false: Medication reviews are solely the responsibility of a pharmacist.
Assign mandatory training and keep all your records in-one-place.

Help and Feedback
Ausmed Education is a Trusted Information Partner of Healthdirect Australia. Verify here .
The systematic review: an overview
Affiliation.
- 1 Edoardo Aromataris is the director of synthesis science at the Joanna Briggs Institute in the School of Translational Health Science, University of Adelaide, South Australia. Alan Pearson is the former executive director and founder of the Joanna Briggs Institute. Contact author: Edoardo Aromataris, [email protected]. The authors have disclosed no potential conflicts of interest, financial or otherwise. The Joanna Briggs Institute aims to inform health care decision making globally through the use of research evidence. It has developed innovative methods for appraising and synthesizing evidence; facilitating the transfer of evidence to health systems, health care professionals, and consumers; and creating tools to evaluate the impact of research on outcomes. For more on the institute's approach to weighing the evidence for practice, go to http://joannabriggs.org/jbi-approach.html.
- PMID: 24572533
- DOI: 10.1097/01.NAJ.0000444496.24228.2c
This article is the first in a new series on systematic reviews from the Joanna Briggs Institute, an international collaborative supporting evidence-based practice in nursing, medicine, and allied health fields. The purpose of the series is to show nurses how to conduct a systematic review-one step at a time. This first installment provides a synopsis of the systematic review as a scientific exercise, one that influences health care decisions.
- Academies and Institutes
- Data Collection* / methods
- Decision Making
- Evidence-Based Practice*
- Nursing Research* / methods
- Systematic Reviews as Topic*
- Research article
- Open access
- Published: 17 January 2017
Effectiveness of medication review: a systematic review and meta-analysis of randomized controlled trials
- Victor Johan Bernard Huiskes 1 ,
- David Marinus Burger 2 ,
- Cornelia Helena Maria van den Ende 3 &
- Bartholomeus Johannes Fredericus van den Bemt 1 , 2 , 4
BMC Family Practice volume 18 , Article number: 5 ( 2017 ) Cite this article
17k Accesses
153 Citations
26 Altmetric
Metrics details
Medication review is often recommended to optimize medication use. In clinical practice it is mostly operationalized as an intervention without co-interventions during a short term intervention period. However, most systematic reviews also included co-interventions and prolonged medication optimization interventions. Furthermore, most systematic reviews focused on specific patient groups (e.g. polypharmacy, elderly, hospitalized) and/or on specific outcome measures (e.g. hospital admissions and mortality). Therefore, the objective of this study is to assess the effectiveness of medication review as an isolated short-term intervention, irrespective of the patient population and the outcome measures used.
A literature search was performed in MEDLINE, EMBASE and Web of Science from their inception through September 2015. Randomized controlled trials (RCTs) with medication review as isolated short term intervention (<3 months) were included. There were no restrictions with regard to patient characteristics and outcome measures. One reviewer extracted and a second checked data. The risk of bias of studies was evaluated independently by two reviewers. A best evidence synthesis was conducted for every outcome measure used in more than one trial. In case of binary variables a meta-analysis was performed in addition to the best evidence synthesis, to quantify the effect.
Thirty-one RCTs were included in this systematic review (55% low risk of bias). A best evidence synthesis was conducted for 22 outcome measures. No effect of medication review was found on clinical outcomes (mortality, hospital admissions/healthcare use, the number of patients falling, physical and cognitive functioning), except a decrease in the number of falls per patient. However, in a sensitivity analysis using a more stringent threshold for risk of bias, the conclusion for the effect on the number of falls changed to inconclusive. Furthermore no effect was found on quality of life and evidence was inconclusive about the effect on economical outcome measures. However, an effect was found on most drug-related problems: medication review resulted in a decrease in the number of drug-related problems, more changes in medication, more drugs with dosage decrease and a greater decrease or smaller increase of the number of drugs.
Conclusions
An isolated medication review during a short term intervention period has an effect on most drug-related outcomes, minimal effect on clinical outcomes and no effect on quality of life. No conclusion can be drawn about the effect on economical outcome measures. Therefore, it should be considered to stop performing cross-sectional medication reviews as standard care.
Peer Review reports
In order to reduce the number of preventable adverse drug events and hospital admissions, medication review is often recommended, incorporated in several guidelines and also frequently reimbursed by health care insurers in various countries [ 1 – 10 ]. Medication review is defined by the Pharmaceutical Care Network Europe (PCNE) as “a structured evaluation of a patient‘s medicines with the aim of optimising medicines use and improving health outcomes. This entails detecting drug related problems and recommending interventions” [ 11 ]. In clinical practice, for each individual patient, medication review is mostly operationalized as an isolated intervention during a short term intervention period [ 5 , 6 , 9 , 12 , 13 ].
Several systematic reviews and meta-analyses already examined the effectiveness of medication review and these did not unequivocally prove the effectiveness of medication review [ 14 – 23 ]. However, these systematic reviews did not only include trials assessing the effect of medication review in terms of how it is mostly operationalized in practice: an isolated cross-sectional assessment of total medication use during a short term intervention period less than 3 months. Most trials in the systematic reviews assessed the effect of medication review as part of multi-faceted pharmaceutical care interventions, consisting of for instance transitional care, adherence counseling and education of patients and healthcare professionals, besides medication review. Such interventions also often last longer than 3 months. Furthermore, most systematic reviews focus on specific patient groups (e.g. polypharmacy, elderly, hospitalized) and/or on specific outcome measures (e.g. hospital admissions and mortality). As a result, more insight is necessary in the effectiveness of medication review as an isolated short-term intervention on clinical outcomes, quality of life, drug-related and economical outcomes.
Therefore, this systematic review aims to summarize the evidence of medication reviews as performed in clinical practice, irrespective of patient characteristics, setting and outcome measures.
This systematic review, assessing the effectiveness of medication review, irrespective of the outcome measures used, follows the PRISMA-guidelines [ 24 , 25 ].
Data sources and searches
A literature search was performed in MEDLINE, EMBASE and Web of Science from their inception through September 2015. For the development of the search strategy and the full electronic search, see Additional file 1 .
Study selection
The inclusion criteria were operationalized based on the PICO model. No restrictions were set concerning the P (patients) and O (outcome measures): interventions could be conducted in any setting and there were no restrictions with regard to patient characteristics and outcome measures. The I (intervention) had to be medication review, which was defined as follows: a structured cross-sectional assessment of a patient’s total medication use leading to recommendations that had to be discussed with the patient and/or clinician within 3 months, in order to improve safety, efficacy or cost-effectiveness. Medication review had to be the single intervention; co-interventions with potential impact on the outcome measures (e.g. discharge counseling, transitional care, non-pharmacological interventions) were not allowed. The C (comparison) was defined as usual care. In addition to PICO the following study selection criteria were formulated: trials had to be randomized controlled trials (RCTs) and only full-length articles were considered for inclusion in this review. Two reviewers independently selected titles/abstracts and the corresponding full text articles to be included in this systematic review. Discrepancies in judgment were discussed in order to reach consensus (VH-BvdB) about final inclusion.
Data extraction and risk of bias assessment
Relevant data on study characteristics and outcomes were extracted by one reviewer (VH) and checked by a second reviewer (NW). P -values ≤0.05 were considered as statistically significant.
Two reviewers independently assessed the risk of bias of the studies eligible for inclusion by using the checklist with criteria for risk of bias from the Cochrane Back Review Group [ 26 , 27 ]. To determine whether a study had a low risk of bias (LRB) or a high risk of bias (HRB), a consensus (VH-BvdB) based scorings method was developed based on the risk of bias assessment.
The twelve Cochrane criteria [ 26 , 27 ] were designated essential (4) or non-essential (8) in relation to research on medication review by a consensus discussion (VH-BvdB). Essential criteria were: was the method of randomization adequate?; Was the drop-out rate described and acceptable?; Were the groups similar at baseline regarding the most important prognostic indicators?; Were co-interventions avoided or similar?. To be considered a study with a low risk of bias, all the essential Cochrane criteria had to be scored positive, whereas a total of at least 6 of the 12 criteria (50%) had to be scored positive. A cutoff of 50% was chosen, as it is not feasible for medication review trials to score positive on certain criteria, like: “was the patient blinded to the intervention”; “was the care provider blinded to the intervention”; “was the outcome assessor blinded to the intervention”. Discrepancies in judgment were discussed in order to reach consensus (VH-BvdB) about the designation of low or high risk of bias for each criterion for each study. If for a specific study an “unclear risk of bias” was scored for the same criterion by both reviewers, the criterion was designated “high risk of bias. The inter-rater agreement of the assessment of risk of bias was assessed by calculating the Cohen’s kappa.
A sensitivity analysis was performed regarding a more stringent cut-off point for risk of bias. The actually used cut-off point for risk of bias was compared with a threshold of ≥8 (2/3 of the attainable 12) of the criteria to be scored positive for a study to be considered a study with a low risk of bias.
Data synthesis and analysis
An adapted version from previously published best evidence syntheses [ 28 , 29 ] was conducted for every outcome measure used in more than one trial, combining a) the percentage of intervention patients included in studies showing effect on the outcome measure and b) the risk of bias of the set of trials using the outcome measure.
The following methodology was used for this purpose:
First, for each outcome measure, the percentage of intervention patients included in studies showing effect on the outcome measure was calculated
The risk of bias of a set of studies per outcome measure was subsequently determined as follows: if 50% or more of the intervention patients included in trials using the outcome measure had a low risk of bias, the set of studies was designated overall low risk of bias
Finally, both the percentage of intervention patients included in studies showing effect on the outcome measure and the risk of bias score for the set of trials per outcome measure were combined to conclude whether medication review has effect on the outcome measure by using the method depicted in Fig. 1 .
Schematic representation of the best evidence synthesis. Schematic representation of the best evidence synthesis, combining a ) the percentage of intervention patients included in studies showing effect on the outcome measure and b ) the risk of bias of the set of trials using the outcome measure. For details: see Additional file 4
In case of binary variables a meta-analysis was performed in addition to the best evidence synthesis, to quantify the effect. In these meta-analyses, effect sizes of binary variables were pooled using their weighted average for the treatment effect (using a random-effect meta-analysis method). Forest plots were created with STATA version 13.1 to summarize the risk ratio (RR) and the 95% confidence interval (CI). Heterogeneity across studies was assessed using I 2 statistics (studies with an I 2 > 50% were considered heterogeneous). Outcome measures reported in only one trial were reported descriptively.
A sensitivity analysis was performed with regard to the impact of large trials with a high risk of bias, on every individual outcome measure. In this sensitivity analysis, large trials with a high risk of bias, with a number of intervention patients greater than the median number of intervention patients per outcome measure, were excluded from the best evidence synthesis.
The literature search provided a total of 13,870 potentially relevant publications which were screened for eligibility. After screening titles and abstracts, 154 articles were left for full text screening. After this screening, 31 RCTs met the inclusion criteria and were included in this systematic review. A flow diagram of the literature search is represented in Fig. 2 .
Flow diagram of the literature search and study selection process
An overview of the study characteristics of the included studies is depicted in Table 1 . Most studies were conducted in primary care (52%), sample size ranged from 64 to 2014 patients, the observation period ranged from 0 to 12 months and 17 (58%) of the trials were conducted in Europe and 7 (23%) in the United States. Patients were involved in the medication reviews in 21 of the 31 studies, the mean age (as reported in 24/31 trials) and the number of drugs (as reported in 18/31 studies) of the intervention patients in each trial ranged from 51.4 to 87.0 years and from 4 to 14 drugs, respectively.
Seventeen studies (55%) met the criteria for low risk of bias. The inter-rater agreement between the two assessors of risk of bias was 0.74 (Cohen’s Kappa). Most common reasons for designating studies high risk of bias were methodological shortcomings on “compliance”, “treatment allocation concealment”, “blindness of patient, care provider and outcome assessor”, “randomization”, “similarity of study groups at baseline” and “drop-out rate”.
- Clinical outcomes
As summarized in Fig. 3 , no effect of medication review was found on clinical outcomes, except for a decrease in the number of falls.
Effect of medication review on clinical outcome measures as assessed in more than 1 trial. The percentage of intervention patients is shown on the y-axis. The black part of the bar represents the percentage of intervention patients included in a trial with a positive effect on a specific outcome measure. The outcome measures, the number of trials using the specific outcome measure, the overall risk of bias of the set of evidence per outcome measure and the conclusion of the best evidence synthesis are shown on the x –axis. T = trials; LRB = low risk of bias; HRB = High risk of bias; inconcl. = inconclusive
Eleven trials (overall low risk of bias, including 2403 intervention patients) assessed the effect of medication review on mortality (for details, see Additional file 2 : Table S1). Data were pooled in a meta-analysis (Additional file 2 : Figure S1) and with a RR of 0.94 (CI, 0.76–1.17) no effect of medication review on mortality was found. Moderate heterogeneity was found between the trials (I 2 = 22.0%, P = 0.234).
Hospital admissions and healthcare use
Data of 11 trials (Additional file 2 : Table S2), including 2041 intervention patients, showed evidence with a low risk of bias for no effect of medication review on the number of hospital admissions (including emergency admissions and visits). Meta-analysis of data from five trials with overall low risk of bias, including 2000 intervention patients, assessing the effect of medication review on the number of patients admitted to the hospital revealed no effect, with a RR of 0.94 (0.82, 1.08) and with moderate heterogeneity (I 2 = 42.3%, P = 0.139) (Additional file 2 : Figure S2 and Table S3). The same applies to the time to first (re)admission in three trials with low risk of bias, including 518 intervention patients, except for a subgroup with only emergency department visits or a low baseline risk for hospital admission (Additional file 2 : Table S4) [ 30 , 31 ]. In addition, no effect of medication review was found on the length of hospital stay in seven trials with overall high risk of bias, including 1330 intervention patients and the number of emergency admissions/visits in seven trials with overall low risk of bias, including 1243 intervention patients (Additional file 2 : Tables S5 to S6). Furthermore, no effect of medication review was demonstrated on the number of General Practitioner (GP) visits in 6 trials with low risk of bias including 1582 intervention patients and on the number of outpatient visits in four trials with overall low risk of bias, including 1144 intervention patients (Additional file 2 : Tables S7 to S8). The meta-analysis of data of 2 trials with overall high risk of bias, including 825 intervention patients, found no effect on the number of patients admitted to residential homes with a RR of 1.17 (0.79, 1.74), with limited heterogeneity (I 2 = 0.0%, p = 0.997) (Additional file 2 : Figure S3 and Table S9).
No best evidence synthesis could be conducted for a variety of other healthcare use related outcome measures used in only one trial. In six trials no effect was found on these outcome measures [ 30 , 32 – 37 ], whereas in two trials an effect was found only in a subdomain of healthcare use related outcome measures or a subgroup of patients [ 32 , 38 ] and in one trial a positive effect was found in favor of patients receiving usual care [ 30 ].
It was observed in two trials with overall low risk of bias, including 467 intervention patients, that medication review decreases the number of falls per patient (Additional file 2 : Table S10). Data of four trials with overall low risk of bias, including 929 intervention patients, were pooled in a meta-analysis (Additional file 2 : Figure S4). This meta-analysis suggested that medication review decreases the number of patients falling (RR 0.68 (0.52, 0.90); I 2 = 41.0%, p = 0.166). However, the best evidence synthesis was inconclusive about the effect on the number of patients falling (Additional file 2 : Table S11). Furthermore, a significant lower fall rate per 1000 patient days (only assessed by Michalek et al) due to medication review was found [ 39 ].
Health status, physical and cognitive outcome measures
Three trials with low risk of bias, including 499 intervention patients, showed no effect of medication review on physical functioning using the Barthel index (Additional file 2 : Table S12). This was confirmed in one study, using three different outcome measures for physical functioning [ 40 ].
Medication review neither improved clinical status [ 41 ], health status [ 40 ] and patient’s perception of severity of illness [ 41 ]. In one study, however, a smaller decrease in self-rated health due to medication review was found [ 35 ].
Two trials, with overall low risk of bias, including 449 intervention patients, found no effect of medication review on cognitive functioning, using the Standard Mini Mental State Examination (Additional file 2 : Table S13). Medication review also did not affect cognitive functioning, expressed with other outcome measures [ 40 , 42 , 43 ], except for the Chrichton-Royal Behaviour Rating Scale [ 42 ].
- Quality of life
The effect of medication review on quality of life is outlined in Fig. 4 . There is evidence with overall low risk of bias that medication review has no effect on quality of life, as measured with the EQ-5D score (based on six trials, including 1583 intervention patients) or the SF-36 score (based on two trials, including 547 intervention patients), whereas evidence with overall high risk of bias was inconclusive about the effect of medication review on the EQ5D-VAS (used in five trials, including 798 intervention patients) (Additional file 2 : Table S14). Pit et al also found no effect of medication review on quality of life measured with the SF-12 score [ 44 ].
Effect of medication review on quality of life, drug-related outcome measures and economical outcome measures as assessed in more than one trial. The percentage of intervention patients is shown on the y-axis. The black part of the bar represents the percentage of intervention patients included in a trial with a positive effect on a specific outcome measure. The outcome measures, the number of trials using the specific outcome measure, the overall risk of bias of the set of evidence per outcome measure and the conclusion of the best evidence synthesis are shown on the x-axis. T = trials; LRB = low risk of bias; HRB = High risk of bias; inconcl. = inconclusive
Drug-related outcome measures
The effect of medication review on drug-related outcome measures is represented in Fig. 4 . An effect of medication review was found on most drug-related outcome measures (the number of drugs, the number of drug changes, the number of drug-related problems and the number of drugs with a dosage decrease), but not on the number of drugs with dosage increase.
Drug-related problems
In four trials with overall high risk of bias, including 599 intervention patients, medication review decreases the number of drug-related problems (Additional file 2 : Table S15). The results of two trials assessing the effect of medication review on the number of patients with drug-related problems (with different pre-defined drug-related problems per trial) were conflicting [ 45 , 46 ].
Number of drug changes and number of drugs with a dosage decrease or increase
Data of three trials with low risk of bias, including 965 intervention patients, showed an increase of the number of drug changes as a result of medication review (Additional file 2 : Table S16). Two other trials with overall high risk of bias, including 486 intervention patients, found an increase of the number of drugs with a dosage decrease, whereas no difference was found with regard to the number of drugs with dosage increase (Additional file 2 : Tables S17 to S18).
Number of drugs and doses
Twelve studies with overall low risk of bias, including 1972 intervention patients, found that medication review leads to a greater decrease or smaller increase of the number of drugs used (Additional file 2 : Table S19). Sellors et al, however, found no difference in the absolute number of drugs used after 5 months due to medication review [ 37 ]. Furthermore, no effect of medication review was found on the number of individual doses per day [ 47 ] and the dosing frequency per day [ 48 ].
Other drug-related outcome measures
Various outcome measures, only used in one trial, but covering the same outcome domains, could not be incorporated in a best evidence synthesis. Two studies assessing the effect of medication review on adherence and knowledge found conflicting results [ 41 , 47 ]. Results with regard to appropriate prescribing and medication use were also conflicting. In two trials, medication review did not improve a set of predefined indicators of prescription quality [ 44 , 46 ], whereas other trials showed improvement of (part of) the indicators [ 38 , 39 , 49 ]. Trials reporting the effect of medication review on scores for appropriateness of prescribing and medication use also found conflicting results. Although medication review improved prescribing appropriateness as measured with the Medication Appropriateness Index (MAI) and the Assessment of Underutilization of Medication Index (AOU) [ 49 ], no effect was found on a composite score reflecting appropriate prescribing of benzodiazepines, NSAIDs and thiazide diuretics [ 44 ]. Finally, the effect of medication review on adverse effects was inconclusive, as one trial demonstrated that medication review decreases adverse effects [ 50 ] and a second trial did not show a significant effect [ 47 ].
- Economical outcomes
Figure 4 shows the effect of medication review on drug costs. Based on the data of nine trials with overall low risk of bias, including 2511 intervention patients, no conclusion could be drawn about the effect of medication review on drug costs (Additional file 2 : Table S20). Trials using various other outcome measures for drug and supply costs did generally not observe effect of medication review on costs [ 37 , 51 , 52 ], except for one study demonstrating that medication review might decrease drug and supply costs due to discontinuation [ 51 ]. Inconclusive results were also observed with respect to total healthcare costs, as two studies found a positive effect of medication review on total healthcare costs [ 32 , 53 ], one study found a temporary positive effect [ 38 ] and two studies did not find any effect [ 37 , 43 ]. Besides this, Burns et al found no decrease or increase of costs related to non-drug GP visits, in patient days, outpatient visits, domiciliary visits and primary care visits due to medication review [ 32 ].
Sensitivity analyses
The sensitivity analysis with a more stringent threshold for risk of bias (≥8; 2/3 of the attainable 12) yielded similar results except for the number of falls per patient, which changed from effective to inconclusive, see Additional file 3 . Based on the sensitivity analysis excluding large trials with high risk of bias from the best evidence synthesis, twice the conclusion changed from effective to inconclusive (number of drug-related problems (DRPs) and number of drugs), twice from inconclusive to not effective (number of patients falling and drug costs), once from not effective to inconclusive (number of emergency admissions) and once from inconclusive to a decreased quality of life (EQ-5D VAS), see Additional file 4 .
This is the first systematic review exploring the effect of medication review as an isolated intervention without co-interventions during a short term (≤3 months) intervention period (as advocated in most medication review guidelines [ 4 – 10 ] and operationalized in practice). Furthermore this systematic review provides an overview of all outcome measures and selection criteria without exclusion criteria based on patient characteristics. In this study, a beneficial effect of medication review was found on most drug-related outcome measures. However, minimal effect was observed on clinical outcomes, no effect was found on quality of life and evidence was inconclusive concerning the effect on economical outcome measures. Only seventeen trials (55%) were designated low risk of bias.
The findings of this systematic review are in line with the findings of other systematic reviews assessing the effect of medication review, although these systematic reviews used other inclusion criteria. Previously published systematic reviews often focused on specific patients (e.g. elderly or hospitalized patients etc.) and/or included trials with multifaceted interventions and/or limited the scope to specific outcome measures.
First of all, the lack of effect of medication review on clinical outcomes (e.g. mortality, number of hospital admissions) observed in this systematic review is in line with the findings of other systematic reviews [ 16 – 22 ], although Patterson found conflicting results concerning hospital admissions [ 14 ]. In other systematic reviews a positive effect of medication review on some clinical outcomes was suggested only when non RCTs [ 21 ], unpublished data [ 18 ], co-interventions [ 15 , 18 ] and/or lengthier interventions (> 3 months) [ 21 ] were included. Secondly, no effect of medication review on quality of life was found by this systematic review, which is also confirmed by other systematic reviews [ 14 , 16 , 17 , 21 , 23 ]. Thirdly, the effect of medication review on drug-related outcomes (e.g. a decrease in the number of drug-related problems and the number of drugs) found in this systematic review was confirmed by other systematic reviews [ 17 , 19 ], although Patterson found no consistent intervention effect on medication-related problems across studies [ 14 ]. In addition, in these systematic reviews an effect of medication review on some other drug-related outcome measures (e.g. adherence, adverse drug events, medication appropriateness) was reported [ 14 – 17 , 19 , 21 , 23 ]. Finally, based on this systematic review, no conclusion could be drawn about the effect of medication review on economical outcome measures , including drug costs . These results were confirmed by the majority of other systematic reviews, since only one out of six other systematic reviews [ 23 ] reported effect of medication review on certain subdomains of economical outcome measures [ 15 – 17 , 19 , 21 , 23 ].
Thus, when the effect of medication review is assessed in terms of how it is operationalized in practice (with medication review as isolated intervention) and even when this effect is assessed irrespective of the patient population and on all available outcome measures, the impact found on clinical outcomes and quality of life is minimal, the observed effect on drug-related outcomes is limited and the evidence about the effect on economical outcome measures is inconclusive. This requires further elaboration of the possible explanations of these findings. Several aspects seem to contribute to these findings, including the 1) selection of patients , the 2) interventions (how medication reviews are being operationalized in practice) and the 3) outcome measures and follow-up time used in trials assessing the effect of medication review. Besides these explanations it might also be the case that the hypothesis that medication review significantly improves clinical outcomes, economical outcomes and quality of life should be rejected.
A possible explanation for the lack of evidence about the effect of medication review is that the 1) selection of patients does not fit the aim of the intervention. If the aim of medication review is, for example, decreasing mortality or preventing patients from being admitted to the hospital, one should select a population with high risk for any of these events. Inclusion criteria often mentioned in medication review trials are age 65-plus and a minimum number of drugs used. Although age and polypharmacy are predominantly positively associated with the risk of having drug-related problems [ 54 – 59 ], several other risk factors (e.g. co-morbidity, renal impairment, high risk medication) contributing to the occurrence of DRPs and/or hospital admissions are found in literature [ 54 , 60 – 69 ]. This suggests that a more sensitive selection of patients for medication review in order to reduce the risk of hospital admission and or death may increase the chance of demonstrating an effect of medication review on these outcomes. Consequently, another aim of the intervention (e.g. increasing adherence) will require a different selection of patients (e.g. lack of therapeutic effect, adherence scores). A second explanation for the lack of evidence about the effect of medication review might be the heterogeneity of 2) the interventions . No golden standard exists for how medication review should be operationalized in practice. Several implicit as well as explicit medication review methods are used [ 70 ]. Furthermore, different levels of medication review are applied in daily practice [ 10 ]. This limits the ability to compare the results of trials assessing the effect of medication review. In addition, the multidisciplinary character of medication reviews is possibly a complicating factor. Often problems are difficult to solve 1) as many care-practitioners are involved and 2) as it is not always clear which healthcare practitioner should be addressed and/or 3) as the responsible physician may not agree with implementation of a recommendation made by another healthcare practitioner. Once the aims of medication review are known, one or more consistent (international) definitions and accompanying operationalizations of medication review should be put into practice. Uniform medication reviews are easier to compare in systematic reviews, this will contribute to the ability to demonstrate effect of these interventions. Finally, the lack of evidence about the effect of medication review might be explained by 3) the outcome measures and follow-up time used in trials assessing the effect of medication review. The outcome measures used in published RCTs examining the effect of medication review are often broad outcome measures, as for instance hospital admissions and all-cause mortality, which are affected by multiple (also not drug related) factors. Although in RCTs these outcome measures may be the ideal outcome measures, since these reflect the overall benefit/risk ratio of drug treatment, no effect of medication review on these outcome measures is found, possibly because the intervention medication review is not powerful enough to have impact on hospitalizations and mortality. Therefore (clinical) outcome measures should be chosen which fit 1) the aim of the medication review (improve safety and (cost-)effectiveness of a patient’s medication use) and 2) are more disease/medication specific (e.g. blood pressure, HbA1c) [ 12 , 71 ]. However, these more disease/medication specific outcome measures should not only reflect the negative effects, but also the positive effects of drug treatment. Although it is often seen in medication review trials, only reporting drug-related outcome measures (e.g. DRPs, number of drugs, adverse events) is suboptimal, as these outcome measures only focus on the disadvantages of drug treatment. Furthermore the outcome measures used are often heterogeneous, as for each outcome a different set of outcome measures is used per trial. This limits the ability to draw robust conclusions. Standardization of outcome measures and time of follow-up should be applied in order to increase the ability to compare the results of trials assessing the effect of medication review. For instance, as one of the aims of the intervention is to improve the quality of life of patients, a standard set of quality of life scores (e.g. EQ-5D and SF-36) should be defined and subsequently used in future research to measure the effect of medication review on quality of life.
In the meantime, it is also conceivable that even when medication review is operationalized and/or investigated as described above, it is not effective on clinical outcomes, economical outcomes and quality of life. A possible explanation is that medication review is a cross-sectional intervention at an arbitrary moment during patient’s drug therapy. However, it might be assumed that at specific moments of drug therapy (e.g. when drugs are started, adapted or stopped) the risk for preventable drug-related problems causing negative clinical outcomes is higher. These specific high-risk moments seem to be the best occasion to apply medication optimization in order to prevent clinically relevant drug-related problems. It can therefore be suggested to redesign the cross-sectional medication review to longitudinal medication therapy management, directly from the start of a drug, targeting at specific risk moments [ 72 ]. Furthermore a more integral approach of pharmaceutical care will give room for medication improvement strategies to shift from a system repairing overdue maintenance to a more individualized approach. Problems related to prescribing according to general guidelines should be solved by means of population based interventions like for instance clinical rules. Other interventions should be developed to address issues related to a patient’s use of medication in the context of his medical condition. For instance individualized medication coaching consults with non-adherent patients or patients experiencing drug-related problems or adverse events.
A couple of limitations are associated with this systematic review. In order to provide a broad overview on the literature about the effect of medication review, no inclusion criteria were applied with regard to outcome measures. Consequently, in the best evidence syntheses, both trials using a specific outcome measure as primary outcome measure and trials using the outcome measure as secondary outcome measure were included. This possibly leads to underpowered trials being part of the best evidence synthesis (BES). However, large trials (with more power) have more impact in the BES. Furthermore, in the best evidence synthesis, it is theoretically possible that a large trial with a high risk of bias has decisive impact on both the overall risk of bias of a set studies and the conclusion about the effect of medication review on a specific outcome measure. However, only in 1/22 best evidence syntheses would the conclusion change to effect (EQ-5D VAS), when studies with a high risk of bias with a number of intervention patients greater than the median number of intervention patients of the trials would be excluded from the best evidence synthesis. Finally a limitation might be the fact that only RCTs were included in this systematic review, although it was a deliberate choice not to include observational studies, as a randomized controlled trial is the most appropriate study design to demonstrate effect of an intervention.
Besides these limitations, some remarks can be made with regard to the robustness of the conclusions. Firstly, only 55% of the included studies were designated a low risk of bias, which results in a smaller body of evidence. In a sensitivity analysis, increasing the threshold for the risk of bias assessment to an arbitrary 2/3 of the attainable maximum score, the percentage of trials with low risk of bias decreased to 39%. For medication review trials, however, on the one hand it is reasonable to relax the threshold to some extent when it comes to blindness of the patient, care provider and outcome assessor. On the other hand this may lead to an overestimation of positive findings of assessor dependent outcome measures, for instance when a non-blinded assessor has to assess whether an outcome is drug-dependent or not. Secondly, the variety of the included patients and settings in this systematic review should be considered. Although no exclusion criteria based on patient characteristics may have resulted in more power, this also may have led to false negative results in subgroups. In other systematic reviews, however, often no effect was found in these subgroups.
Although an isolated medication review during a short term intervention period (how it is mostly operationalized in practice) has an effect on most drug-related outcomes, medication review has minimal effect on clinical outcomes, no effect on quality of life and no conclusion could be drawn about the effect on economical outcome measures. Therefore, it should be considered to stop performing cross-sectional medication reviews as standard care. It may also be considered to shift the focus of research from cross-sectional medication review to other strategies to improve the safety and (cost-)effectiveness of drug treatment. If, despite this, research on the effect of cross sectional medication review is still continued, high quality studies including high-risk patients and using relevant outcome measures should be conducted to assess if/when medication reviews can contribute to better medication use and subsequent better clinical outcomes. However, more effort should be put in the development and evaluation of other medication improvement strategies, like more individualized and longitudinal medication therapy management, targeting at specific risk moments of drug treatment and targeting at problems that patients experience themselves.
Abbreviations
Assessment of underutilization of medication index
Confidence interval
Drug related problem
General practitioner
High risk of bias
Low risk of bias
Medication appropriateness index
Pharmaceutical care network Europe
Randomized controlled trial
Beijer HJ, de Blaey CJ. Hospitalisations caused by adverse drug reactions (ADR): a meta-analysis of observational studies. Pharm World Sci. 2002;24:46–54.
Article CAS PubMed Google Scholar
Leendertse AJ, Visser D, Egberts AC, van den Bemt PM. The relationship between study characteristics and the prevalence of medication-related hospitalizations: a literature review and novel analysis. Drug Saf. 2010;33:233–44.
Article PubMed Google Scholar
Al Hamid A, Ghaleb M, Aljadhey H, Aslanpour Z. A systematic review of hospitalization resulting from medicine-related problems in adult patients. Br J Clin Pharmacol. 2014;78:202–17.
Medicines optimisation: the safe and effective use of medicines to enable the best possible outcomes. http://www.nice.org.uk/guidance/NG5/chapter/1-recommendations#/medication-review . Accessed 1 Feb 2016.
Multidisciplinary guideline polypharmacy in the elderly 2012 (NHG). https://www.nhg.org/sites/default/files/content/nhg_org/uploads/polyfarmacie_bij_ouderen.pdf . Accessed 1 Feb 2016.
Grundsatzpapier zur Medikationsanalyse und zum Medikationsmanagement. http://www.abda.de/fileadmin/assets/Medikationsmanagement/Grundsatzpapier_MA_MM_GBAM.pdf . Accessed 1 Feb 2016.
Guidance on the medicines use review service. http://www.nhsemployers.org/case-studies-and-resources/2012/09/guidance-on-the-medicines-use-review-service . Accessed 1 Feb 2016.
Clinical Medication Review, A Practice Guide. http://www.cumbria.nhs.uk/ProfessionalZone/MedicinesManagement/Guidelines/MedicationReview-PracticeGuide2011.pdf . Accessed 1 Feb 2016.
Shaw J, Seal R, Pilling M. Room for review: a guide to medication review 2002. http://www.webarchive.org.uk/wayback/archive/20140627113046/http://www.npc.nhs.uk/review_medicines/intro/resources/room_for_review.pdf . Accessed 1 Feb 2016.
Clyne W, Blenkinsopp A, Seal R. A guide to medication review National Prescribing Centre 2008. http://www.webarchive.org.uk/wayback/archive/20140627113046/http://www.npc.nhs.uk/review_medicines/intro/resources/agtmr_web1.pdf . Accessed 1 Feb 2016.
Position Paper on the PCNE definition of Medication Review 2016. http://www.pcne.org/upload/files/149_Position_Paper_on_PCNE_Medication_Review_final.pdf . Accessed 25 July 2016.
Blenkinsopp A, Bond C, Raynor DK. Medication reviews. Br J Clin Pharmacol. 2012;74:573–80.
Article PubMed PubMed Central Google Scholar
Medication Therapy Management in Pharmacy Practice. Core Elements of an MTM Service Model. http://www.pharmacist.com/sites/default/files/files/core_elements_of_an_mtm_practice.pdf . Accessed 20 July 2016.
Patterson SM, Cadogan CA, Kerse N, Cardwell CR, Bradley MC, Ryan C, et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev. 2014;10:CD008165.
Google Scholar
Tan EC, Stewart K, Elliott RA, George J. Pharmacist services provided in general practice clinics: a systematic review and meta-analysis. Res Social Adm Pharm. 2014;10:608–22.
Graabaek T, Kjeldsen LJ. Medication reviews by clinical pharmacists at hospitals lead to improved patient outcomes: a systematic review. Basic Clin Pharmacol Toxicol. 2013;112:359–73.
Holland R, Desborough J, Goodyer L, Hall S, Wright D, Loke YK. Does pharmacist-led medication review help to reduce hospital admissions and deaths in older people? A systematic review and meta-analysis. Br J Clin Pharmacol. 2008;65:303–16.
Christensen M, Lundh A. Medication review in hospitalised patients to reduce morbidity and mortality. Cochrane Database Syst Rev. 2016;2:CD008986.
PubMed Google Scholar
Alldred DP, Raynor DK, Hughes C, Barber N, Chen TF, Spoor P. Interventions to optimise prescribing for older people in care homes. Cochrane Database Syst Rev. 2013;2:CD009095.
Wallerstedt SM, Kindblom JM, Nylen K, Samuelsson O, Strandell A. Medication reviews for nursing home residents to reduce mortality and hospitalization: systematic review and meta-analysis. Br J Clin Pharmacol. 2014;78:488–97.
Hatah E, Braund R, Tordoff J, Duffull SB. A systematic review and meta-analysis of pharmacist-led fee-for-services medication review. Br J Clin Pharmacol. 2014;77:102–15.
Hohl CM, Wickham ME, Sobolev B, Perry JJ, Sivilotti ML, Garrison S, et al. The effect of early in-hospital medication review on health outcomes: a systematic review. Br J Clin Pharmacol. 2015;80:51–61.
Viswanathan M, Kahwati LC, Golin CE, Blalock SJ, Coker-Schwimmer E, Posey R, et al. Medication therapy management interventions in outpatient settings: a systematic review and meta-analysis. JAMA Intern Med. 2015;175:76–87.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100.
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647.
van Tulder M, Furlan A, Bombardier C, Bouter L. Updated method guidelines for systematic reviews in the cochrane collaboration back review group. Spine (Phila Pa 1976). 2003;28:1290–9.
van Tulder MW, Assendelft WJ, Koes BW, Bouter LM. Method guidelines for systematic reviews in the Cochrane Collaboration Back Review Group for Spinal Disorders. Spine (Phila Pa 1976). 1997;22:2323–30.
Article Google Scholar
Vriezekolk JE, van Lankveld WG, Geenen R, van den Ende CH. Longitudinal association between coping and psychological distress in rheumatoid arthritis: a systematic review. Ann Rheum Dis. 2011;70:1243–50.
Zwikker HE, van den Bemt BJ, Vriezekolk JE, van den Ende CH, van Dulmen S. Psychosocial predictors of non-adherence to chronic medication: systematic review of longitudinal studies. Patient Prefer Adherence. 2014;8:519–63.
Lisby M, Bonnerup DK, Brock B, Gregersen PA, Jensen J, Larsen ML, et al. Medication review and patient outcomes in an orthopedic Department: a randomized controlled study. J Patient Saf. 2015. Epub ahead of print.
Zillich AJ, Snyder ME, Frail CK, Lewis JL, Deshotels D, Dunham P, et al. A randomized, controlled pragmatic trial of telephonic medication therapy management to reduce hospitalization in home health patients. Health Serv Res. 2014;49:1537–54.
Burns A, Furniss L, Cooke J, Craig SKL, Scobie S. Pharmacist medication review in nursing homes: A cost analysis. Int J Geriatr Psychopharmacol. 2000;2:137–41.
Heselmans A. Medication review by a clinical pharmacist at the transfer point from ICU to ward: A randomized controlled trial. J Clin Pharm Ther. 2015;40:578–83.
Holland R, Lenaghan E, Harvey I, Smith R, Shepstone L, Lipp A, et al. Does home based medication review keep older people out of hospital? The HOMER randomised controlled trial. BMJ. 2005;330:293.
Lenander C, Elfsson B, Danielsson B, Midlov P, Hasselstrom J. Effects of a pharmacist-led structured medication review in primary care on drug-related problems and hospital admission rates: a randomized controlled trial. Scand J Prim Health Care. 2014;32:180–6.
Lisby M, Thomsen A, Nielsen LP, Lyhne NM, Breum-Leer C, Fredberg U, et al. The effect of systematic medication review in elderly patients admitted to an acute ward of internal medicine. Basic Clin Pharmacol Toxicol. 2010;106:422–7.
CAS PubMed Google Scholar
Sellors J, Kaczorowski J, Sellors C, Dolovich L, Woodward C, Willan A, et al. A randomized controlled trial of a pharmacist consultation program for family physicians and their elderly patients. CMAJ. 2003;169:17–22.
PubMed PubMed Central Google Scholar
Bond CM, Fish A, Porteous TH, Reid JP, Scott A, Antonazzo E. A randomised controlled trial of the effects of note-based medication review by community pharmacists on prescribing of cardiovascular drugs in general practice. Int J Pharm Pract. 2007;15:39–46.
Michalek C, Wehling M, Schlitzer J, Frohnhofen H. Effects of “Fit fOR The Aged” (FORTA) on pharmacotherapy and clinical endpoints--a pilot randomized controlled study. Eur J Clin Pharmacol. 2014;70:1261–7.
Williams ME, Pulliam CC, Hunter R, Johnson TM, Owens JE, Kincaid J, et al. The short-term effect of interdisciplinary medication review on function and cost in ambulatory elderly people. J Am Geriatr Soc. 2004;52:93–8.
Lim WS, Low HN, Chan SP, Chen HN, Ding YY, Tan TL. Impact of a pharmacist consult clinic on a hospital-based geriatric outpatient clinic in Singapore. Ann Acad Med Singapore. 2004;33:220–7.
Furniss L, Burns A, Craig SK, Scobie S, Cooke J, Faragher B. Effects of a pharmacist’s medication review in nursing homes. Randomised controlled trial. Br J Psychiatry. 2000;176:563–7.
Pope G, Wall N, Peters CM, O’Connor M, Saunders J, O’Sullivan C, et al. Specialist medication review does not benefit short-term outcomes and net costs in continuing-care patients. Age Ageing. 2011;40:307–12.
Pit SW, Byles JE, Henry DA, Holt L, Hansen V, Bowman DA. A quality use of medicines program for general practitioners and older people: a cluster randomised controlled trial. Med J Aust. 2007;187:23–30.
Meredith S, Feldman P, Frey D, Giammarco L, Hall K, Arnold K, et al. Improving medication use in newly admitted home healthcare patients: a randomized controlled trial. J Am Geriatr Soc. 2002;50:1484–91.
Olsson IN, Runnamo R, Engfeldt P. Drug treatment in the elderly: an intervention in primary care to enhance prescription quality and quality of life. Scand J Prim Health Care. 2012;30:3–9.
Jameson J, VanNoord G, Vanderwoud K. The impact of a pharmacotherapy consultation on the cost and outcome of medical therapy. J Fam Pract. 1995;41:469–72.
Zermansky AG, Petty DR, Raynor DK, Lowe CJ, Freemantle N, Vail A. Clinical medication review by a pharmacist of patients on repeat prescriptions in general practice: a randomised controlled trial. Health Technol Assess. 2002;6:1–86.
Gallagher PF, O’Connor MN, O’Mahony D. Prevention of potentially inappropriate prescribing for elderly patients: a randomized controlled trial using STOPP/START criteria. Clin Pharmacol Ther. 2011;89:845–54.
Jameson JP, VanNoord GR. Pharmacotherapy consultation on polypharmacy patients in ambulatory care. Ann Pharmacother. 2001;35:835–40.
Britton ML, Lurvey PL. Impact of medication profile review on prescribing in a general medicine clinic. Am J Hosp Pharm. 1991;48:265–70.
Sellors C, Dalby DM, Howard M, Kaczorowski J, Sellors J. A pharmacist consultation service in community-based family practices: a randomized, controlled trial in seniors. J Pharm Technol. 2001;17:264–9.
Pacini M, Smith RD, Wilson EC, Holland R. Home-based medication review in older people: is it cost effective? Pharmacoeconomics. 2007;25:171–80.
Onder G, Pedone C, Landi F, Cesari M, Della VC, Bernabei R, et al. Adverse drug reactions as cause of hospital admissions: results from the Italian Group of Pharmacoepidemiology in the Elderly (GIFA). J Am Geriatr Soc. 2002;50:1962–8.
Alkema GE, Wilber KH, Simmons WJ, Enguidanos SM, Frey D. Prevalence of potential medication problems among dually eligible older adults in Medicaid waiver services. Ann Pharmacother. 2007;41:1971–8.
Bourgeois FT, Shannon MW, Valim C, Mandl KD. Adverse drug events in the outpatient setting: an 11-year national analysis. Pharmacoepidemiol Drug Saf. 2010;19:901–10.
Goulding MR. Inappropriate medication prescribing for elderly ambulatory care patients. Arch Intern Med. 2004;164:305–12.
Hu SH, Capezuti E, Foust JB, Boltz MP, Kim H. Medication discrepancy and potentially inappropriate medication in older Chinese-American home-care patients after hospital discharge. Am J Geriatr Pharmacother. 2012;10:284–95.
Laroche ML, Charmes JP, Nouaille Y, Fourrier A, Merle L. Impact of hospitalisation in an acute medical geriatric unit on potentially inappropriate medication use. Drugs Aging. 2006;23:49–59.
Leendertse AJ, Egberts AC, Stoker LJ, van den Bemt PM. Frequency of and risk factors for preventable medication-related hospital admissions in the Netherlands. Arch Intern Med. 2008;168:1890–6.
Schuler J, Duckelmann C, Beindl W, Prinz E, Michalski T, Pichler M. Polypharmacy and inappropriate prescribing in elderly internal-medicine patients in Austria. Wien Klin Wochenschr. 2008;120:733–41.
Olivier P, Bertrand L, Tubery M, Lauque D, Montastruc JL, Lapeyre-Mestre M. Hospitalizations because of adverse drug reactions in elderly patients admitted through the emergency department: a prospective survey. Drugs Aging. 2009;26:475–82.
Ruiter R, Visser LE, Rodenburg EM, Trifiro G, Ziere G, Stricker BH. Adverse drug reaction-related hospitalizations in persons aged 55 years and over: a population-based study in the Netherlands. Drugs Aging. 2012;29:225–32.
Fialova D, Topinkova E, Gambassi G, Finne-Soveri H, Jonsson PV, Carpenter I, et al. Potentially inappropriate medication use among elderly home care patients in Europe. JAMA. 2005;293:1348–58.
Onder G, Landi F, Cesari M, Gambassi G, Carbonin P, Bernabei R. Inappropriate medication use among hospitalized older adults in Italy: results from the Italian Group of Pharmacoepidemiology in the Elderly. Eur J Clin Pharmacol. 2003;59:157–62.
Ruggiero C, Dell’Aquila G, Gasperini B, Onder G, Lattanzio F, Volpato S, et al. Potentially inappropriate drug prescriptions and risk of hospitalization among older, Italian, nursing home residents: the ULISSE project. Drugs Aging. 2010;27:747–58.
Claydon-Platt K, Manias E, Dunning T. Medication-related problems occurring in people with diabetes during an admission to an adult teaching hospital: a retrospective cohort study. Diabetes Res Clin Pract. 2012;97:223–30.
O’Neil CK, Poirer TI. Impact of patient knowledge, patient-pharmacist relationship, and drug perceptions on adverse drug therapy outcomes. Pharmacotherapy. 1998;18:333–40.
Wilmer CM, Huiskes VJB, Natsch S, Rennings AJM, van den Bemt BJF, Bos JM. Drug-related problems in a clinical setting: a literature review and cross-sectional study evaluating factors to identify patients at risk. Eur J Hosp Pharm. 2015;22:229–35.
De Smet PA, Denneboom W, Kramers C, Grol R. A composite screening tool for medication reviews of outpatients: general issues with specific examples. Drugs Aging. 2007;24:733–60.
Hinchliffe A. Medicines use review by community pharmacists. http://www2.nphs.wales.nhs.uk:8080/PharmaceuticalPHTDocs.nsf/($All)/49CAA20A63ADF04E802578AA00379DEF/$File/Microsoft%20Word%20-%20Medicines%20use%20review%20by%20community%20pharmacists%20v1%200%20_2_.pdf?OpenElement . Accessed 7 Sep 2016.
van den Bemt BJF, Huiskes VJB. The medication therapy management pyramid shifting medication review to an integrated medication therapy management process. Eur J Hosp Pharm. 2015;22:219–21.
Briggs S, Pearce R, Dilworth S, Higgins I, Hullick C, Attia J. Clinical pharmacist review: a randomised controlled trial. Emerg Med Australas. 2015;27:419–26.
Graffen M, Kennedy D, Simpson M. Quality use of medicines in the rural ambulant elderly: a pilot study. Rural Remote Health. 2004;4:184.
Krska J, Cromarty JA, Arris F, Jamieson D, Hansford D, Duffus PR, et al. Pharmacist-led medication review in patients over 65: a randomized, controlled trial in primary care. Age Ageing. 2001;30:205–11.
Kwint HF, Faber A, Gussekloo J, Bouvy ML. Effects of medication review on drug-related problems in patients using automated drug-dispensing systems: a pragmatic randomized controlled study. Drugs Aging. 2011;28:305–14.
Lenaghan E, Holland R, Brooks A. Home-based medication review in a high risk elderly population in primary care--the POLYMED randomised controlled trial. Age Ageing. 2007;36:292–7.
Mannheimer B, Ulfvarson J, Eklof S, Bergqvist M, Andersen-Karlsson E, Pettersson H, et al. Drug-related problems and pharmacotherapeutic advisory intervention at a medicine clinic. Eur J Clin Pharmacol. 2006;62:1075–81.
Meyer TJ, Van KD, Marsh S, Prochazka AV. Reduction of polypharmacy by feedback to clinicians. J Gen Intern Med. 1991;6:133–6.
Milos V, Rekman E, Bondesson A, Eriksson T, Jakobsson U, Westerlund T, et al. Improving the quality of pharmacotherapy in elderly primary care patients through medication reviews: a randomised controlled study. Drugs Aging. 2013;30:235–46.
Zermansky AG, Alldred DP, Petty DR, Raynor DK, Freemantle N, Eastaugh J, et al. Clinical medication review by a pharmacist of elderly people living in care homes--randomised controlled trial. Age Ageing. 2006;35:586–91.
Download references
Acknowledgments
We would like to thank N.S. (Naomi) Wartenberg for checking data entry of the included studies.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article and its additional files.
Authors’ contributions
VH and BvdB had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: VH, DB and BvdB. Acquisition, analysis, or interpretation of data: VH, CvdE and BvdB. Drafting of the manuscript: VH and BvdB. Critical revision of the manuscript for important intellectual content: VH, DB, CvdE and BvdB. Statistical analysis: VH, CvdE and BvdB. Study supervision: BvdB. All authors have read and approved the final version of this manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Author information, authors and affiliations.
Department of Pharmacy, Sint Maartenskliniek, Hengstdal 3, 6574 NA, Ubbergen, The Netherlands
Victor Johan Bernard Huiskes & Bartholomeus Johannes Fredericus van den Bemt
Department of Pharmacy, Radboud University Medical Center, Geert Grooteplein-Zuid 10, 6525 GA, Nijmegen, The Netherlands
David Marinus Burger & Bartholomeus Johannes Fredericus van den Bemt
Department of Rheumatology, Sint Maartenskliniek, Hengstdal 3, 6574NA, Ubbergen, The Netherlands
Cornelia Helena Maria van den Ende
Department of Clinical Pharmacy and Toxicology, Maastricht University Medical Center +, Maastricht, Peter Debyelaan 15, 6229 HX, Maastricht, The Netherlands
Bartholomeus Johannes Fredericus van den Bemt
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Victor Johan Bernard Huiskes .
Additional files
Additional file 1:.
Development of the search strategy and full electronic search. Full electronic search (MEDLINE). (DOCX 17 kb)
Additional file 2:
Data not shown in the results section of the main text of the manuscript. Table S1. Effect of medication review on mortality. Figure S1. Meta-analysis of the studies assessing the effect of medication review on mortality. Table S3. Effect of medication review on the number of patients with hospital admissions. Figure S2. Meta-analysis of the studies assessing the effect of medication review on the number of patients with hospital admissions. Table S4. Effect of medication review on time to first hospital (re)admission. Table S6. Effect of medication review on the number of emergency admissions/visits. Table S7. Effect of medication review on the number of GP visits. Table S8. Effect of medication review on the number of outpatient visits. Table S9. Effect of medication review on the number of patients admitted to residential homes. Figure S3. Meta-analysis of the studies assessing the effect of medication review on the number of patients admitted to residential homes. Table S10. Effect of medication review on the number of falls per patient. Table S11. Effect of medication review on the number of patients falling. Figure S4. Meta-analysis of the studies assessing the effect of medication review on the number of patients falling. Table S12. Effect of medication review on the Barthel index. Table S13. Effect of medication review on the Standard Mini Mental State Examination. Table S14. Effect of medication review on the quality of life. Table S15. Effect of medication review on the number of drug-related problems. Table S16. Effect of medication review on the number of drug changes. Table S17. Effect of medication review on the number of drugs with a dosage decrease. Table S18. Effect of medication review on the number of drugs with a dosage increase. Table S19. Effect of medication review on the number of drugs. Table S20. Effect of medication review on drug costs. (DOCX 124 kb)
Additional file 3:
Risk of bias assessment: sensitivity analysis. Sensitivity analysis regarding a more stringent cut-off point for risk of bias. (DOCX 19 kb)
Additional file 4:
Best evidence synthesis: sensitivity analysis. Sensitivity analysis with regard to the impact of large trials with high risk of bias on every individual outcome measure in the best evidence synthesis. (DOCX 20 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Huiskes, V.J.B., Burger, D.M., van den Ende, C.H.M. et al. Effectiveness of medication review: a systematic review and meta-analysis of randomized controlled trials. BMC Fam Pract 18 , 5 (2017). https://doi.org/10.1186/s12875-016-0577-x
Download citation
Received : 31 March 2016
Accepted : 26 December 2016
Published : 17 January 2017
DOI : https://doi.org/10.1186/s12875-016-0577-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Medication review
- Drug utilization review[Mesh]
- Pharmaceutical services[Mesh]
- Review[Publication Type]
- Meta-analysis[Publication Type]
- Drug-related outcomes
BMC Primary Care
ISSN: 2731-4553
- General enquiries: [email protected]

IMAGES
COMMENTS
During a structured medication review, take into account: the person's, and their family members or carers where appropriate, views and understanding about their medicines. the person's, and their family members' or carers' where appropriate, concerns, questions or problems with the medicines.
This review aimed to identify, describe and discuss different interventions targeting medication use optimization in nursing homes and to identify research gaps. Finding Prescription of the whole medication regimen or of specific medication classes was the most studied aspect.
Not only does the review encompass a snapshot of the medication list, but it also allows the pharmacist to review key nursing, dietary, and therapeutic activities, rehabilitation progress, and medical notes.
What is a Medication Review? A medication review is a systematic evaluation of a patient’s medicines. It is an interprofessional process that aims to ensure medicines are being used safely and effectively at all times (CEC 2019).
The purpose of the series is to show nurses how to conduct a systematic review-one step at a time. This first installment provides a synopsis of the systematic review as a scientific exercise, one that influences health care decisions.
This is the first systematic review exploring the effect of medication review as an isolated intervention without co-interventions during a short term (≤3 months) intervention period (as advocated in most medication review guidelines [4 – 10] and operationalized in practice).