An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List


The Epidemiology of Obesity: A Big Picture
Adela hruby , phd, mph, frank b hu , md, phd, mph.
- Author information
- Copyright and License information
Corresponding Author and Contact Information Adela Hruby, PhD, MPH, Department of Nutrition, Harvard School of Public Health, 665 Huntington Avenue, Boston, MA 02115, USA, [email protected]
The epidemic of overweight and obesity presents a major challenge to chronic disease prevention and health across the life course around the world. Fueled by economic growth, industrialization, mechanized transport, urbanization, an increasingly sedentary lifestyle, and a nutritional transition to processed foods and high calorie diets over the last 30 years, many countries have witnessed the prevalence of obesity in its citizens double, and even quadruple. Rising prevalence of childhood obesity, in particular, forebodes a staggering burden of disease in individuals and healthcare systems in the decades to come. A complex, multifactorial disease, with genetic, behavioral, socioeconomic, and environmental origins, obesity raises risk of debilitating morbidity and mortality. Relying primarily on epidemiologic evidence published within the last decade, this non-exhaustive review discusses the extent of the obesity epidemic, its risk factors—known and novel—, sequelae, and economic impact across the globe.
1. Introduction
Obesity is a complex, multifactorial, and largely preventable disease ( 1 ), affecting, along with overweight, over a third of the world’s population today ( 2 , 3 ). If secular trends continue, by 2030 an estimated 38% of the world’s adult population will be overweight and another 20% will be obese ( 4 ). In the USA, the most dire projections based on earlier secular trends point to over 85% of adults being overweight or obese by 2030 ( 5 ). While growth trends in overall obesity in most developed countries seem to have leveled off ( 2 ), morbid obesity in many of these countries continues to climb, including among children. In addition, obesity prevalence in developing countries continues to trend upwards toward US levels.
Obesity is typically defined quite simply as excess body weight for height, but this simple definition belies an etiologically complex phenotype primarily associated with excess adiposity, or body fatness, that can manifest metabolically and not just in terms of body size ( 6 ). Obesity greatly increases risk of chronic disease morbidity—namely disability, depression, type 2 diabetes, cardiovascular disease, certain cancers—and mortality. Childhood obesity results in the same conditions, with premature onset, or with greater likelihood in adulthood ( 6 ). Thus, the economic and psychosocial costs of obesity alone, as well as when coupled with these comorbidities and sequealae, are striking.
In this article, we outline the prevalence and trends of obesity, then review the myriad risk factors to which a preventive eye must be turned, and finally present the costs of obesity in terms of its morbidity, mortality, and economic burden.
2. Classification of Body Weight in Adults
The current most widely used criteria for classifying obesity is the body mass index (BMI; body weight in kilograms, divided by height in meters squared, Table 1 ), which ranges from underweight or wasting (<18.5 kg/m 2 ) to severe or morbid obesity (≥40 kg/m 2 ). In both clinical and research settings, waist circumference, a measure of abdominal adiposity, has become an increasingly important and discriminating measure of overweight/obesity ( 7 ). Abdominal adiposity is thought to be primarily visceral, metabolically active fat surrounding the organs, and is associated with metabolic dysregulation, predisposing individuals to cardiovascular disease and related conditions ( 8 ). Per internationally used guidelines of metabolic syndrome—a cluster of dysmetabolic conditions that predispose individuals to cardiovascular disease of which abdominal adiposity is one component—a waist circumference resulting in increased cardiovascular risk is defined as ≥94 cm in European men, and ≥80 cm in European women, with different cut points recommended in other races and ethnicities (e.g., ≥90 and ≥80 cm in men and women, respectively, in South Asians, Chinese, and Japanese) ( 8 , 9 ).
Common Classifications of Body Weight in Adults and Children
Abbreviations used: BMI, body mass index; IOTF, International Obesity Task Force; SD, standard deviation; WHO, World Health Organization; WH weight-for-height; Z, z score.
In the USA, typically “Class” is referred to as “Grade”. Obesity has an unofficial cut point of BMI ≥27 kg/m 2 in Asian populations.
Per WHO 2000 classifications, in BMI as kg/m 2 (139). These categories, if not the exact terminology, of adult weight status have been adopted by other major health organizations, including the US National Heart, Lung, and Blood Institute and National Institute of Diabetes and Digestive and Kidney Diseases (135).
Preobesity has an unofficial cut point of 23-<27 kg/m 2 in Asian populations.
Per WHO 2006 classifications, BMIZ are BMI z scores, and WHZ are WH z scores, based on age- and sex-specific growth standards for children 0-60 months old. In children aged <2 years, weight-for-length is used (10).
Per WHO 2007 classifications, BMIZ are BMI z scores are based on age- and sex-specific growth standards and references for children aged 5-19 years (11).
Per Cole et al. (140), for the IOTF based on age- and sex-specific curves defined to pass through BMIs of 25 or 30 kg/m 2 at age 18, for children aged 2-18 years.
Per CDC 2000 classifications, BMI percentiles are based on age- and sex-specific growth references for children aged 2-19 years (12).
3. Classification of Body Weight in Children
In children, body weight classifications ( Table 1 ) differ from those of adults because body composition varies greatly as a child develops, and further varies between boys and girls primarily owing to differences in sexual development and maturation. The World Health Organization (WHO) Child Growth Standards are the most widely currently used classification system of weight and height status for children from birth to 5 years old, based on data from children in six regions across the globe born and raised in optimal conditions ( 10 ). In 2007, the WHO published updated growth references combining the 1977 National Center for Health Statistics (NCHS)/WHO growth reference and the 2006 WHO Child Growth Standards to create the most recent BMI-for-age references for individuals aged 5–19 years ( 11 ). Thus, the latest WHO guidelines are designed to represent relatively seamless standards and references from birth all the way into late adolescence/early adulthood.
In the USA, the Centers for Disease Control and Prevention (CDC) currently use the 2000 CDC growth references based on 1963–1994 US children’s data, to determine age- and sex-specific BMI percentiles for children aged 2–19 years ( 12 ). Overweight is defined in US children as age- and sex-specific BMI ≥85th and <95th percentile, while obesity is ≥95th percentile ( 13 ). Cut points for severe obesity in childhood have been proposed in recognition of the alarming growing prevalence of this extreme condition, defined as the 99th BMI percentile ( 13 ) or 120% of the 95th percentile ( 14 ). For US children <2 years old, the CDC currently uses the 2006 WHO Child Growth Standards, described above ( 15 ).
4. Prevalence and Trends
4.1. adult obesity—us and europe.
The first indications that obesity was taking on epidemic proportions originated in the USA and Europe. With few restrictions on access to or availability of food, the prevalence of overweight and obesity in the USA climbed virtually unmitigated over the last 50 years. Today, those who are overweight (BMI 25–<30 kg/m 2 ) or obese (BMI ≥30 kg/m 2 ) in the USA eclipse two-fold the numbers of those who are normal weight ( 16 ). In US adults, 1960–1994 trends showed that while levels of overweight hovered at approximately 31% over the time period, in contrast, age-adjusted obesity jumped from 13 to 23%, bringing the crude prevalence of overweight or obesity to 55% of the American population ( 17 ). Unfortunately, 1994 did not represent the endpoint of the upward trend, as the following decade saw adult obesity rise from 23 to 32% by 2003–2004 ( 16 ). In the last 10 years, national estimates of obesity seem to indicate that the steady upward trend of obesity in Americans has leveled off at a prevalence of about 35% ( 16 ) ( Figure 1 ), perhaps having reached some “Malthusian” obesity limit. However, certain subpopulations are faring worse than others, as 2011–2012 obesity rates in Hispanics and non-Hispanic blacks were 43 and 48%, respectively, pointing to a disproportionate burden in differing racial/ethnic and/or socioeconomic status (SES) groups. Gender also plays a role, with women being disproportionately affected by extreme obesity (classes 2–3, BMI ≥35 kg/m 2 ) than men, regardless of age or race/ethnicity ( 16 ).
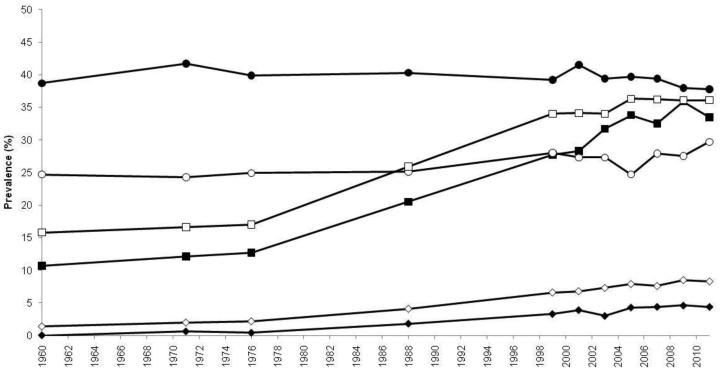
Trends in age-adjusted prevalence of overweight, obesity, and extreme obesity in US adults, aged 20–74 years, 1960–2012. Trends in prevalence of overweight as BMI 25–<30 kg/m 2 (circles), and upward trends in obesity as BMI ≥30 kg/m 2 (squares), and extreme obesity as BMI ≥40 kg/m 2 (diamonds) in adult males (closed points) and females (open points). The figure is based on data from NHES I (1960–1962), NHANES I (1971–1974), NHANES II (1976–1980), NHANES III (1988–1994), and NHANES (1999–2000, 2001–2002, 2003–2004, 2005–2006, 2007–2008, 2009–2010, 2011–2012). Data derived are derived from Ogden, et al ., and Fryar, et al . ( 16 , 141 ). BMI, body mass index; NHANES, National Health and Nutrition Examination Survey; NHES, National Health Examination Survey.
Meanwhile, in Europe, longitudinal data (1992–1998 to 1998–2005) from participants in five countries involved in the European Prospective Investigation into Cancer and Nutrition (EPIC) study (Italy, the United Kingdom, the Netherlands, Germany, and Denmark), indicate that adult obesity increased modestly from 13 to 17% in that time period ( 18 ). However, were such linear trends were to continue, the overall obesity prevalence in these populations could reach 30% by 2015, paralleling US rates. A more conservative projection suggests a prevalence of just 20% obesity in these populations by 2015, if public awareness and public health measures take hold ( 18 ).
European studies including populations beyond EPIC indicate there is considerable disparity in overweight/obesity between European countries. A systematic review of national and regional surveys conducted between 1990 and 2008 points to obesity rates as low as 4.0 and 6.2% in French men and women, respectively (regional survey, 1994–1996), and as high as 30.0 and 32.0% in Czech men and women, respectively (national survey, 2002–2005) ( 19 ). Regional trends within Europe are apparent, with southern Italy and southern Spain, and Eastern European countries showing higher prevalence of obesity than countries in Western and Northern Europe ( 19 ). As in the USA, these data suggest that socioeconomic disparities and relatively recent/ongoing economic transitions are playing a considerable role in apparent differences across and within countries with respect to obesity risk.
4.2. Child Obesity—USA and Europe
US children may be faring better than their adult counterparts in some ways ( 16 ), potentially offsetting earlier dire predictions of rampant obesity by 2030 ( 5 ). In national surveys, levels of overweight in children, as in adults, seem to have leveled off (or even declined) at approximately 30% of US children aged 2–19 years ( 16 , 20 ). However, this belies a potentially disturbing long-term trend in the rising prevalence of extreme obesity (equivalent to adult class 2 obesity and higher, BMI ≥35 kg/m 2 ). Since 1999–2000, the prevalence of class 2 obesity in children (BMI ≥120% of the 95th percentile) has risen from 3.8 to 5.9% and class 3 obesity (BMI ≥140% of the 95th percentile) has doubled from 0.9 to 2.1%, the latter category jumping 30% since 2009–2010 alone ( 20 ). Again, as in their adult counterparts, certain sub-populations appear to be faring worse than others, notably Hispanic girls and Black boys, in whom overweight, obesity, and class 2 obesity have increased significantly ( 20 ).
Childhood obesity prevalence varies considerably between and within countries as well. Relatively recent estimates based on 2007–2008 data of children aged 6–9-years collected in 12 European countries as a part of the WHO European Childhood Obesity Surveillance Initiative observed overweight/obesity (BMI z score >+1 standard deviation [SD]) prevalence of 19.3–49.0% of boys and 18.4–42.5% of girls, while obesity (BMI z score >+2 SD) affected 6.0–26.6% of boys and 4.6–17.3% of girls. Researchers continued to observe the trend of north-south and west-east gradients evident in adults, with the highest levels of overweight in southern European countries ( 21 ).
4.3. Obesity Beyond North America and Europe
The data discussed above focus on the USA and European countries, many with robust national health surveillance programs. While historical data tends to be scarcer outside of these regions, an alarming picture has emerged over the last decades in low- and middle-income countries around the globe, complicated by rapidly changing socioeconomic environments. While country-specific trends are not discussed in this article, regional and national estimates of long-term changes in child (<20 years old) and adult (>20 years old) overweight and obesity have increased in nearly all countries and regions since 1980 ( Figure 2 ) ( 2 , 3 ). While the USA still may boast the largest absolute numbers of overweight and obese individuals, several other nations exceed the USA in terms of overall prevalence and, moreover, the rate of growth in certain countries is disheartening. For example, the prevalence of overweight and obesity in nationally representative Mexican adults was estimated to be 71.3% overweight/obese, with overweight at 38.8% and obesity at 32.4% ( 22 ). This prevalence represents an increase of 15% since 2000, placing this population among the most rapidly accelerating in terms of obesity prevalence over the last decade. Further, while rates of overweight remained relatively stable since 2000 at approximately 38% overall, extreme obesity (class 3, BMI ≥40 kg/m 2 ) increased by an estimated 76.5% from 2000 to 2012. These trends are also evident in countries outside of the Americas. In China, for example, between 1993 and 2009, overweight (BMI 25 to <27.5 kg/m 2 ) doubled in men (8 to 17%) and increased from 11 to 14% in women. Meanwhile, obesity (BMI ≥27.5 kg/m 2 ) nearly quadrupled in men, from 3 to 11%, and doubled in women, from 5 to 10%. Chinese children are faring as badly as their adult counterparts: overweight/obesity doubled from 6 to 13% in children aged 6–17 years over the same time period, suggesting that the obesity epidemic will continue to deepen in this country ( 23 ).

Prevalence of overweight and obesity in adults aged ≥20 years by global region, 1980–2008. From left to right, each column represents the estimated regional prevalence of overweight and obesity for 1980, 1985, 1990, 1995, 2000, 2005, and 2008. For a given region, a dark gray column indicates the lowest estimated prevalence in the trend, while the highest estimated prevalence is indicated by a black column. As is evident, the vast majority of regions demonstrate the lowest estimated prevalence of overweight and obesity in 1980, and the highest in 2008, demonstrating the global reach of obesity. The scale shows 25, 50, and 100% prevalence columns, for reference. Asterisks denotes high income. Data are sourced from Stevens, et al . ( 3 ).
5. Risk Factors for Obesity
Currently, our greatest gap in knowledge is not regarding the numbers of risk factors, nor in their independent impact on risk, but rather in how they interact with one another—their confluence—to produce today’s aptly if unfortunately named “globesity” epidemic. Obesity arises as the result of an energy imbalance between calories consumed and the calories expended, creating an energy surplus and a state of positive energy balance resulting in excess body weight. This energy imbalance is partially a result of profound social and economic changes at levels well beyond the control of any single individual. These “obesogenic” changes—economic growth, growing availability of abundant, inexpensive, and often nutrient-poor food, industrialization, mechanized transportation, urbanization—have been occurring in high-income countries since the early 20th century, and today these forces are accelerating in low- and middle-income countries. And yet, not all of us living in obesogenic environments experience the same growth in our waistlines. Hereditary factors—genetics, family history, racial/ethnic differences—and our particular socioeconomic and sociocultural milieus have been shown to affect risk of obesity ( Table 2 ) even in ostensibly similar obesogenic environments. So while body weight regulation is and should be viewed as a complex interaction between environmental, socioeconomic, and genetic factors, ultimately, personal behaviors in response to these conditions continue to play a dominant role in preventing obesity. Importantly, apart from genetics, every risk factor discussed below is modifiable .
Risk Factors, Comorbidities, and Sequelae of Obesity
5.1. Genetics of Obesity
To date, over 60 relatively common genetic markers 1 have been implicated in elevated susceptibility to obesity ( 24 , 25 ); however, the 32 most common genetic variants are thought to account for <1.5% of the overall inter-individual variation in BMI ( 24 ). When these 32 “top” genetic hits are combined into a genetic risk of obesity score, those with the highest genetic risk (i.e., carriers of over 38 risk alleles), have just a 2.7 kg/m 2 higher BMI on average than those with a low genetic risk. This translates into about a 15-lb (7-kg) weight difference between two 5’3” (160 cm) individuals with high versus low genetic risk ( 24 ). Although genetics undoubtedly play a role, this relatively small difference in BMI, coupled with the dramatic rise in obesity over the last half century in developed and developing nations alike point to obesity risk factors beyond genetics. A concomitant and rich area of research has therefore evolved investigating gene-environment interaction based on the idea that underlying genetic risk predisposes individuals to particularly adverse (or beneficial) effects of behavioral or environmental exposures such as diet and exercise, a concept scientifically popularized in, for example, the “thrifty gene” hypothesis ( 26 ). In many ways, these types of gene-environment interactions are playing out in population research: for example, a variant in FTO (rs9939609)—the strongest obesity susceptibility locus—increases odds of obesity in risk allele carriers by an estimated 23% per allele; however, this risk is modified by physical activity in adults ( 27 , 28 ) and children ( 29 ), among other factors. Nevertheless, these types of interactions have so far been investigated in relatively few genetic risk loci out of millions, and with just a handful of environmental factors, raising important questions of how to aggregate this complexity for public health and ultimately personalized medicine.
In addition, parental diet, lifestyle, and other exposures have been implicated in subsequent offspring obesity risk, including famine exposure ( 30 ), parental obesity ( 31 – 33 ), smoking ( 34 ), endocrine-disrupting and other chemicals ( 35 , 36 ), and weight gain during gestation and gestational diabetes ( 33 , 37 ). These and other studies point to lasting effects of fetal programming that via differing mechanisms, likely epigenetic, result in substantial repercussions in life course health, with implications across the socioeconomic/food availability spectrum. Careful management of diet and lifestyle in pre- and perinatal periods could exert a considerable impact on the obesity epidemic for generations to come ( 37 ).
5.2. Individual Behaviors
5.2.1. diet.
In the decades preceding the 21st century, the vast majority of research on obesity risk factors focused on individual-level, largely modifiable behaviors. The role of diet and physical activity in mitigating obesity risk and reducing prevalent obesity have received the most attention, and with good reason: 15% of deaths in 2000 in the USA were attributable to excess weight, owing to poor diet and physical inactivity ( 38 ). Caloric intake and expenditure needed for weight maintenance or healthy growth has historically taken center stage ( 39 ), and caloric restriction remains today a primary focus of most popular and clinical weight-management and weight-loss approaches.
Beyond overall caloric intake to regulate body weight, a tremendous amount of research has attempted to resolve the roles of diet quality and dietary patterns, including those specifying combinations of macronutrients ( 40 ). Evidence from clinical trials have almost universally shown that caloric restriction, regardless of dietary pattern, is associated with better weight outcomes ( 40 ). Although the metabolic nuances and relative merits of the differing dietary patterns for various comorbid conditions are still being investigated, the evidence seems to suggest that merely adhering to a diet—nearly irrespective of what type of healthy diet it is—has an impact on weight loss/control ( 41 – 43 ).
For long-term maintenance of healthy weight, evidence from observational cohorts indicate that diets that are considered “healthier” lead to better long-term weight maintenance, or at least mitigate weight gain typically associated with aging through middle age. For example, research in US health professionals pointed to averaged 4-year weight gain throughout middle age as being strongly associated with increasing intake of potato chips and potatoes, sugar-sweetened beverages, and processed and unprocessed red meats, but inversely associated with the intake of vegetables, fruits, whole grains, nuts, and yogurt ( 44 ). Specific food groups, such as sugar-sweetened beverages, have received considerable attention largely because added sugar consumption (primarily as sugar-sweetened beverages) has been rising concomitantly with prevalent obesity ( 45 ). Indeed, the weight of the evidence about the role of sugar-sweetened beverages in obesity ( 46 , 47 ) is a strong impetus for public health interventions and policies, such as limiting advertising on these beverages as in Mexico ( 48 ), attempts to limit beverage sizes permitted for sale as in New York City ( 49 ), taxation, eliminating sale in schools, etc.
5.2.2. Physical Activity, Sedentary Behaviors, and Sleep
Personal behaviors beyond diet (physical activity, sleep, sedentary and screen time, and stress) have also been independently associated with weight change and maintenance in adulthood. Combined with diet, these elements have synergistic and likely cumulative effects on an individual’s ability to maintain or obtain a healthy body weight over the life course. Recently reviewed evidence from randomized trials and observational studies support 2008 US recommendations for weight management ( 50 ), consistently showing that in general, 150–250 minutes per week of moderate intensity activity is required to prevent weight gain, or aid in weight loss when accompanied by dietary restriction ( 51 ). Activity (>250 minutes per week) is associated with weight loss and weight maintenance after weight loss ( 51 ). Leisure-time activities involving sitting, but which are not truly restful behaviors, such as getting <6 or >8 hours of sleep in adults and adolescents ( 44 , 52 – 55 ) or <10–11 hours of sleep in children ( 52 ), television viewing or screen time ( 44 , 56 , 57 ), and other leisure-time sitting ( 58 ) are also associated with weight gain.
5.3. Socioeconomic Risk Factors: Income and Education
Income has had a shifting role in obesity risk over the last century. As late as the mid-20th century, the USA and Europe could link wealth directly with obesity—the wealthier an individual, the more likely to be overweight. Over the last few decades, however, perhaps owing to the abundance of cheap and highly available food, coupled with changing sociocultural norms, this link has flipped. Today, wealth in the USA tends to be inversely correlated with obesity, and it is those who are at or below the level of poverty who appear to have the highest rates of obesity ( 59 ). Indeed, in US cities where the homeless are surveyed, the prevalence of overweight and obesity parallels that of non-homeless populations, contrary to our typical beliefs about thinness accompanying food insecurity or homelessness ( 60 , 61 ).
More broadly, across 11 Organisation for Economic Co-Operation and Development (OECD) countries, SES, whether defined by household income or occupation-based social class, showed an inverse relationship with obesity: women, in particular, had consistently higher prevalence of overweight/obesity the less affluent they were ( 62 ). In men, too, those in low income strata tended to have higher prevalence of obesity, but the gradient for overweight reversed in about half of the countries surveyed. That is, in some countries, poverty was associated with more prevalent overweight than wealth, but in others, lower income was associated with more favorable weight status. The differences between sexes in terms of income status and obesity, in particular the trend reversal in men, may be in part due to low-paying jobs typically involving more physically demanding work performed by men more than by women ( 62 ). Adding complexity to this picture is the role of education: in the 11 OECD countries discussed above, education showed a strong inverse relationship with overweight/obesity, particularly in women, who had consistently higher prevalence of overweight/obesity the less educated they were ( 62 ).
As wealth rises in low- and middle-income countries, it is expected for poverty-obesity patterns to begin more closely mimicking those of high-income countries. Evidence of this transition is already accumulating. In explorations of the role of education and wealth in women and weight status in four middle-income countries (Colombia, Peru, Jordan, and Egypt), authors observed a significant interaction between education and wealth: in women with little or no education, higher income was associated with 9–40% higher odds of obesity, while in those with higher levels of education, the association with income was either not present (Egypt, Peru) or associated with 14–16% lower odds of obesity (Jordan, Colombia) ( 63 ). This suggests that in currently transitioning economies, education may offset the apparently negative effects of increasing purchasing power in emerging obesogenic environments. However, the protective effect of education has yet to be seen in the poorer countries, such as India, Nigeria, and Benin, where both education and wealth were directly associated with increased odds of obesity ( 63 ).This is perhaps unsurprising, as obesity was relatively rare at <6.0% of women in these countries, and >50% of women had little or no education.
The glimmer of hope, then, is that in the context of a paradigm of diseases of affluence, in which the transition to wealth seem to invariably lead to higher obesity and thus greater chronic disease burden, higher education levels may yet offset some of the frightening challenges that lay before us.
5.4. Environmental 2 Risk Factors
5.4.1. the built environment.
Research on the built environment tends to focus on a few measurable characteristics of neighborhoods as they relate to weight status, while holding sociodemographic and other person-level characteristics constant. Such neighborhood characteristics range from more concrete factors (e.g., fast food restaurants, supermarkets, parks, transportation, etc.) to more variably scored factors (e.g., walkability, neighborhood healthiness). Most studies of the built environment have been cross-sectional, tending to focus on one or two characteristics; thus, findings on the relative importance or effects of given characteristics on obesity have been inconsistent ( 66 – 72 ), revealing the fundamental challenge of teasing out whether neighborhood characteristics play a causal role in weight status, or whether health-minded folks inhabit health-friendly areas to begin with (residential selection bias) ( 73 ). However, the emerging picture points to the primacy of diet-related built environments over those associated with physical activity. While presence of neighborhood physical activity or recreational spaces has been associated with increased physical activity levels or energy expenditure ( 71 , 72 ), healthy food environments, characterized by availability of produce or presence of supermarkets over convenience stores or fast food restaurants, play a potentially more important role ( 68 , 70 , 74 , 75 ).
Research on the causality of the built environment as obesity-inducing or health-promoting is critical for municipalities and public health authorities to justify potentially costly improvements to public spaces and/or zoning regulations. There is an unmet need for standardized measures, definitions, and criteria, established residential and occupational geographic radii relevant to health, and research methodologies that can take into account the complexity of something as seemingly simple as a neighborhood.
5.4.2. Environmental “Pathogens”: Viruses, Microbiomes, and Social Networks
Growing evidence from animal and human studies indicates that obesity may be attributable to infection, or that obesity itself may be a contagion. Infectious agents include viruses, the trillions of microbiota inhabiting the human gut, and, of course, obese humans as infectious agents themselves.
Although several viruses have been identified as potentially having a causal role in obesity ( 76 ), Ad-36 is among the most studied, being causally associated with adiposity in animals. Studies in diverse human populations generally support greater Ad-36 viral loads as probably causal of obesity in both children and adults ( 76 – 79 ), with links to other metabolic traits ( 77 , 79 ).
Ground-breaking research in the last decade has emerged on the role of trillions of gut bacteria—the human microbiome—in relation to obesity, energy metabolism, and carbohydrate and lipid digestion, opening promising therapeutic avenues for obesity and disease ( 80 ). Two primary phyla of bacteria differ in their proportions in lean vs. obese populations; these proportions change as obese individuals lose weight and correlate highly with the percentage of body weight lost ( 81 ). Broad and sometimes dramatic changes in microbiome populations have been catalogued following gastric bypass surgery ( 80 ), and in both the short- ( 82 , 83 ) and long-term ( 81 , 83 ) in response to changes in dietary composition ( 80 ). Research in mice indicates that increased adiposity is a transmissible trait via microbiome transplantation ( 84 ), and has prompted similar experimental fecal transplantation research in humans for the promotion of weight loss ( 85 ). In addition, other research has examined the feeding of pre- and probiotics as therapeutic modalities designed to manipulate the gut microbiome; these strategies also show promise for a range of conditions ( 85 ).
Finally, the importance of social networks—real and virtual—in obesity is a fascinating, relatively new area of research that capitalizes on known characteristics of infectious disease transmission. In a landmark 2007 study examining the spread of obesity due to social ties using 32-year prospective data from the Framingham Heart Study, Christakis and Fowler ( 86 ) showed that an individual’s chances of becoming obese increased by 57% if he or she had a friend who became obese in a given 4-year interval. This was a stronger risk ratio than that observed between pairs of adult siblings or even between spouses. Conversely, it may be possible to capitalize on the social contagion of obesity in the reverse direction, that is, in the promotion of healthy weight and behavior. Intervention studies of weight loss often include a social-relational component, although the evidence supporting any single approach or its efficacy is relatively scarce ( 87 ). In theory, a supportive network, community, or coaching relationship is supposed to improve weight loss; despite a lack of strong evidence, it is a key component of many popular commercial (e.g., Weight Watchers), trial/intervention, and online approaches.
6. Costs of Obesity: Co-Morbidities, Mortality, and Economic Burden
Obesity is associated with concomitant or increased risk of nearly every chronic condition, from diabetes, to dyslipidemia, to poor mental health. Its impacts on risk of stroke and cardiovascular disease, certain cancers, and osteoarthritis are significant.
6.1. Overall Mortality
In the year 2000 in the USA, 15% of deaths were attributable to excess weight, owing to poor diet and physical inactivity ( 38 ). Overweight/obesity in middle age shortens life expectancy by an estimated 4–7 years ( 88 ). Many long-term cohort studies, as well as three recent major syntheses of pooled data from established cohorts ( 89 – 91 ), which adequately accounted for history of smoking and chronic disease status, unequivocally show that overweight and obesity over the life course is associated with excess risk of total mortality, death from cardiovascular disease, diabetes, cancer, or accidental death ( 89 – 97 ).
Some studies suggest that excess body weight may be protective against mortality from certain chronic conditions—resulting in a so-called “obesity paradox.” However, most studies that have shown an obesity paradox, or no association between obesity and mortality, have been conducted in groups of older (>65) or elderly patients or in those with chronic conditions, or have inadequately accounted for smoking. Indeed, the role of excess adiposity in old age is unclear. While the protective effects of overweight in specific instances of diseased older populations may be real, these observations are fraught with methodological problems, especially reverse causation, and belie the limitations of generalizing excess adiposity’s supposed benefits to younger populations over the life course, not least because excess body weight leads to higher disease incidence to begin with ( 7 ).
6.2. Diabetes
Excess weight and diabetes are so tightly linked that the American Diabetes Association recommends physicians test for type 2 diabetes and assess risk of future diabetes in asymptomatic people ≥45 years old simply if they are overweight/obese, and regardless of age if they are severely obese ( 98 ). Overweight raises risk of developing type 2 diabetes by a factor of three, and obesity by a factor of seven, compared to normal weight ( 99 ). Excess weight in childhood and in young adulthood, and weight gain through early to mid-adulthood are strong risk factors for diabetes ( 100 – 102 ). While not every overweight/obese individual has diabetes, some 80% of those with diabetes are overweight/obese ( 103 ). Obesity itself raises diabetes risk even in the absence of other metabolic dysregulation (insulin resistance, poor glycemic control, hypertension, dyslipidemia). While metabolically healthy obese individuals are estimated to have half the risk of their metabolically unhealthy counterparts, they still have four times the risk of those who are normal weight and metabolically healthy ( 104 ).
6.3. Heart and Vascular Diseases
Ischemic heart disease and stroke are the leading causes of death in the USA and globally ( 105 ). Excess body weight is a well-known risk factor for heart disease and ischemic stroke, including their typical antecedents—dyslipidemia and hypertension. Recent studies have consistently shown that benign obesity appears to be a myth ( 106 – 108 ); overweight clearly adds to risk of heart disease and stroke beyond its implications for hypertension, dyslipidemia, and dysglycemia.
Given childhood obesity rates, research has lately focused on the role of obesity in early life and subsequent adulthood disease. Obesity in childhood or adolescence has been associated with twofold or higher risk of adult hypertension, coronary heart disease, and stroke ( 100 ). A recent study pooling data from four child cohorts (aged 11 years at baseline with average 23-year follow-up), observed that, compared with individuals who were normal weight in childhood and non-obese as adults, those who were normal weight or overweight but became obese as adults, or who were obese and stayed obese into adulthood, had considerably higher risk of high-risk dyslipidemia, hypertension, and higher carotid intima-media thickness. Notably, those individuals who were overweight/obese as children, but non-obese as adults, had similar risk profiles to those individuals who were never obese, indicating that the potential health effects of childhood obesity can be offset by weight loss prior to or while entering into adulthood ( 109 ).
6.4. Cancer
An estimated 6% of all cancers (4% in men, 7% in women) diagnosed in 2007 were attributable to obesity ( 110 ). Beyond being a major risk factor for diabetes, which itself is a risk factor for most cancers, obesity has long been understood to be associated with increased risk of esophageal, colon, pancreatic, postmenopausal breast, endometrial, and renal cancers ( 111 ). More recently, evidence has accumulated that overweight and/or obesity raise risk of cancers of the gallbladder ( 112 ), liver ( 113 ), ovaries (epithelial) ( 114 ), and advanced cancer of the prostate ( 115 ), as well as leukemia ( 116 ).
6.5. Trauma and Infection
A study in Pennsylvania (USA) trauma centers (2000–2009) showed that in-hospital mortality and risk of major complications of surgery were increased in obese patients as compared to non-obese patients. Severely obese patients had upwards of 30% increased risk of mortality from their trauma than non-obese patients, and double the risk of major complications. Severely obese females also had more than double the risk of developing wound complications, and quadruple the risk of developing decubitus ulcers ( 117 ). A recent meta-analysis of obesity in trauma care concluded that obesity was associated with 45% increased odds of mortality, longer stays in the intensive care unit, and higher rates of complications, and tended to associate with longer durations of mechanical ventilation and longer stays in the hospital overall, compared to non-obese patients, despite equivalent injury severity ( 118 ).
While elevated risk of chronic disease is a seemingly obvious consequence of obesity, increasing attention is being given to increased risk of infection and infectious disease in obesity, including surgical-site, intensive care unit (ICU)-acquired catheter, blood, nosocomial, urinary tract, and cellulitis and other skin infections ( 119 ), community-acquired infections, and poorer recovery outcomes owing to higher risk of influenza, pneumonia, bacteremia, and sepsis ( 119 ). Impaired immunological response may be an underlying mechanism; recent research has demonstrated lower vaccine efficacy and serological response to vaccination in the obese. For example, a recent study estimated an eightfold increase in the odds of non-responsiveness to hepatitis-B vaccination in obese versus normal-weight women ( 120 ).
The consequences of a global obesity epidemic may not merely be greater chronic and infectious disease burden for the obese, but also a greater global burden of infectious disease owing to obesity. Greater infectious disease vigilance may be required in populations with high levels of overweight/obesity, and there is a clear need for better clinical practice guidelines (e.g., use and dosage of antimicrobials, vaccines, other pharmaceuticals) for obese individuals.
6.6. Mental Health
The role of weight in mental health faces causal challenges, but what is clear is that obesity and adiposity are associated with anatomical as well as functional changes in the human brain. Studies in older populations have shown that BMI is inversely correlated with brain volume, and that obese older adults, compared to normal weight counterparts, show atrophy in the frontal lobes, anterior cingulate gyrus, hippocampus, and thalamus ( 121 ). In addition, obesity in children and adolescents (aged >9 years) has been associated with smaller orbitofrontal cortex gray matter volume, along with poorer performance in certain domains of executive function (e.g., inhibitory control) ( 122 ). Being overweight in midlife increases risk of Alzheimer's disease, vascular dementia, or any type of dementia by 35, 33, and 26%, respectively; even higher risk is observed for obesity ( 123 ). Importantly, physical activity, even among overweight individuals, may stave off poor mental functioning: moderately active or highly active adult overweight Finns did not have significantly increased risk of poor mental functioning at a 7-year follow-up compared to those who were normal weight and highly active, but inactive and overweight patients presented a nearly 40% increased risk of poor mental functioning ( 124 ). Thus, exercise may play an important mediating role in the relationship between excess body weight and age-related cognitive decline.
6.7. Economic Burden of Obesity
In the USA, recent estimates indicate that obese men are thought to incur an additional US$1,152 per year in medical spending, particularly due to hospitalizations and prescription drugs, compared to their non-obese counterparts, while obese women incur over double that of obese men, an additional US$3,613 per year in medical spending (year 2005 values). Extrapolating these costs to the national level, authors estimate some US$190 billion per year of healthcare spending, approximately 21% of US healthcare expenditures, is due to treating obesity and obesity-related conditions ( 125 ).
Total hospital costs account for a part of this: another author group studied non-bariatric, non-obstetric hospital procedures for obese patients, finding they were US$648 higher (year 2009 values) per capita than for non-obese patients. The estimated national hospital expenditures for the largest volume surgical procedures was US$160 million higher per year for obese than for their non-obese counterparts ( 126 ).
Employers bear a substantial brunt of obesity-related costs in the USA. Data from the Human Capital Management Services Research Reference Database (2001–2012) on employees and their dependents was used to compare medical, drug, sick leave, short-term disability, and workers’ compensation costs as well as absent days across three BMI strata: <27, ≥27–<30, and ≥30 kg/m 2 . Each of the costs was incrementally higher in ascending BMI categories. For example, total annual costs and total days absent in the highest vs. lowest BMI strata were US$6,313 versus US$4,258 (year 2012 values), and 7.5 versus 4.5 days. In addition, productivity was lowest in the obese group ( 127 ).
Finally, lifetime direct incremental medical costs of obesity in childhood in the USA were estimated to range from US$12,660 to US$19,630 (year 2012 values) for an obese 10-year old compared to a normal-weight 10-year old, if expected weight gain through adulthood among the normal weight child occurs ( 128 ). If normal weight children were to not continue on the typical weight gain trajectory into overweight/obesity, estimated incremental medical costs for today’s 10-year old obese child ranges between US$16,310 and US$39,080. Putting these figures into perspective, multiplying the lifetime medical cost estimate of US$19,000 by the number of obese 10-year-olds today generates a total direct medical cost of obesity of roughly US$14 billion for this 10-year old age group alone. In terms of big picture savings, the upper estimate of US$39,000 per case represents two years of public college tuition for that child ( 128 ).
In Europe, a 2008 review of 13 studies in ten Western European countries estimated the obesity-related healthcare burden had a relatively conservative upper limit of €10.4 billion annually (in Germany, in 1995 € equivalent), and ranging between <0.1 to 0.61% of each country’s gross domestic product (GDP). The review relied on study data from as early as the 1980s in the Netherlands, through 2002 in most of the remaining countries surveyed ( 129 ). A more recent review focused on 19 studies published in 2007–2010 in eight Western European countries (predominantly Germany, Denmark, and the United Kingdom). Excess health care costs of obesity or derivations of excess health care costs by comparisons of mean costs between normal weight and obese individuals in seven of the reviewed studies were between €117 and €1,873 per person (based on the € valuation given in each study year). Excess costs increased particularly due to severe obesity. Approximately 23% of medication costs and 6.9% of out-of-pocket costs were attributable to overweight or obesity. Health economic models estimated that 2.1–4.7% of total health care costs and 2.8% of total hospital costs were due to overweight and obesity. Total (direct and indirect) costs were generally unchanged from the 2008 estimate of the earlier review, accounting for 0.47–0.61% of GDP in these countries ( 130 ).
In the context of the Brazilian Unified Health System (i.e., public hospitals), estimated direct costs of diseases related to overweight/obesity in outpatient and inpatient care based on 2008–2010 data were US$2.1 billion annually (year 2010 values), 68.4% of which was attributable to hospitalizations, and the remainder due to ambulatory procedures ( 131 ). The largest costs of outpatient and inpatient care in both sexes were due to cardiovascular disease (US$747 million) followed by overweight- and obesity-related neoplasms (US$299.8 million), asthma (US$34 million), type 2 diabetes (US$3.7 million), and osteoarthritis (US$3.9 million). Authors estimated that these direct costs were a considerable underestimate of the true burden of overweight/obesity in Brazil, which would include private health care expenditures, as well as indirect costs due to lost productivity, premature death, and home care ( 131 ).
Given the predicted rise in obesity in Brazil, coronary heart disease, stroke, hypertension, cancers, osteoarthritis, and diabetes are projected to at least double by 2050, with concomitant doubling in health care costs, from US$5.8 billion in 2010 to US$10.1 billion per year—totaling US$330 billion over 40 years (year 2010 values). It is estimated that a 5% reduction in mean BMI across the population could save Brazil some US$57 billion over that time frame ( 132 ). A similar analytic approach that substituted Mexican prevalence and trends for the Brazilian ones estimated 2010 costs of obesity at US$806 million (year 2000 values), which were projected to increase to US$1.7 billion by 2050, at which point a mere 1% reduction in BMI prevalence in Mexico could save an estimated US$85 million per year ( 133 ).
Of course, none of these estimates include dollars spent on the weight-loss industry, which is estimated to be over US$60 billion dollars in 2014 in the USA alone ( 134 ), and includes non-prescription drugs and supplements, diet plans, gym memberships, workout videos, and an endless stream of money-making schemes.
7. Touching on Solutions, and Some Conclusions
Obesity is a major contributor to preventable disease and death across the globe, and poses a nearly unprecedented challenge not just to those tasked with addressing it at the public health level, or at the healthcare provider level, but to each of us as individuals, for none of us are immune. Increasing ease of life, owing to reduced physical labor and automated transportation, an increasingly sedentary lifestyle, and liberal access to calorie-dense food, driven by dramatic economic growth in many parts of the world in the last century, have turned a once rare disease of the affluent into one of the most common diseases—increasingly of the poor. That barely one in three people in the USA today are normal weight portends, quite simply, an astounding and frightening future. Significant reductions in public health and healthcare expenditures could occur around the world if we were able to stem the tide of childhood obesity trends, and if young and middle-aged overweight and obese adults lost approximately10% of their body weight, as recommended for a considerably reduced risk of debilitating chronic conditions ( 135 ).
Obesity is complex. Although its risk factors are myriad and compounding, there is an urgent need for deeper understanding of the way risk factors interact with each other, and the potential solutions to the epidemic are as multi-leveled and complex as its causes. There are calls for applying systems-level ( 136 ) and systems epidemiology ( 137 ) approaches to this and related nutrition and metabolic diseases, approaches which attempt to comprehensively address biological, behavioral, and environmental contributors to disease as well as their intricate feedback loops. Additional research on solutions to this epidemic would include, for example, examining the relative cost/benefit to individuals and populations of individual versus systemic policies and/or interventions, concurrently or independently, particularly when individuals and communities must decide between approaches given limited resources, and moreover, with the currently limited evidence in the case of broad industry, agricultural, or public health policies. For example, we could attempt to limit national production and import of sugar-sweetened beverages, tax sugar-sweetened beverages, or restrict fast food restaurant zoning. These largely political acts seem relatively inexpensive, but may have economic impacts in communities and regions beyond what we currently understand. We may push for the increasing medicalization of obesity, including developing an obesity vaccine. While such a “cure” may someday arise, the medicalization of a condition typically improves its treatment rather than its prevention, and prevention is key in the case of obesity. However, preventing and remediating obesity in children and adults—e.g., via health and wellness incorporation into curricula at every educational level from kindergarten through medical school—requires vast resources allocated to educators, as well as earlier diagnosis and treatment of overweight (education, counseling, drug treatment, etc.). Given these resource costs, perhaps greater attention should be given to pregnancy, a condition which is already highly medicalized and which may be an ideal preventive avenue for the provision of nutrition education and intensive monitoring of weight gain, to ensure that children have the most optimal start with respect to their future obesity risk. Clearly, no single approach is optimal, but with limited resources, an evidence base supporting one or more approaches or their combination is needed, as is tenacity and perhaps some audacity by local government and public health authorities in testing some of these approaches within their populations. However, an epidemic of this magnitude needs, quite simply, more resources. One of the reasons why the American Medical Association opted to declare obesity a “disease” was to give obesity the label it needs for greater allocation of resources for research, prevention, and treatment ( 1 ).
Despite the many unknowns, we can be cautiously optimistic about our ability to address the obesity epidemic. Indeed, we have relatively successfully faced similarly daunting public health challenges before: smoking, to name just one. While tobacco can loosely be thought of as a single product, and our food culture is infinitely more complex, as a case study in how to approach obesity, it provides numerous lessons in multi-level solutions to a major health threat in terms of both mitigation and prevention. We began by developing an understanding of smoking’s epidemiological impact and the healthcare costs borne by society, uncovered its biological basis, learned about and applied behavior change, and initiated and carried out vast public health, public policy, political, and economic strategies that ultimately affected whole environments as well as sociocultural norms.
It took over half a century to achieve the immense success we have with regard to smoking in the USA and still we are not yet tobacco-free ( 138 ); other parts of the world continue to wrestle with it to a greater degree. It has only been a couple decades since we first deeply appreciated that obesity was epidemic. We clearly still have a long way to go.
Key Points for Decision Makers.
In 2013, an estimated one in three adults worldwide was overweight or obese, and adult obesity exceeded 50% in several countries around the globe. While the prevalence of adult obesity in the developed world seems to have stabilized, the prevalence of obesity in children and adolescents globally, as well as adults obesity in developing countries, is still increasing. In addition, some developed countries continue to observe increasing prevalence of extreme classes of obesity.
Overweight and obesity—defined as excess body weight for height—have genetic, behavioral, socioeconomic, and environmental origins.
Obesity increases risk of major chronic diseases, including heart disease, diabetes, depression, and many cancers, as well as premature death.
Estimates of annual healthcare costs attributable to obesity are US$190 billion per year in the USA, approximately 21% of US healthcare expenditures.
Given its complexity, the obesity epidemic requires multilevel and integrated solutions, from individual intervention, to broad food policy, industry, and agriculture initiatives.
Acknowledgements
The authors declare no conflict of interest. AH is supported by an American Diabetes Association Mentor-Based Postdoctoral Fellowship award. FH is supported by NIH grants DK51158, HL60712, P30 DK46200, and U54CA155626. The authors broadly thank the researchers in this field for their consistent and tireless work in illuminating the etiology, sequelae, and solutions to this complex condition.
See also http://www.genome.gov/gwastudies/
We do not review the impact of food, agriculture, trade, and nutrition policy on obesity in the present paper, but refer interested readers to a recent review ( 64 ). Further, we do not address the body of growing evidence on the role of environmental pollutants–“obesogens”–in obesity, specifically those known as endocrine-disrupting chemicals. We refer readers to recent reviews on the topic ( 35 , 36 , 65 ).
Author Contributions
AH wrote the first draft of the paper. AH and FH contributed to writing, revised, and edited the paper. AH is the final guarantor of this work and takes full responsibility for its contents. Both authors read and approved the final manuscript.
- 1. American Medical Association AMA Adopts New Policies on Second Day of Voting at Annual Meeting [Internet] 2013 [cited 2014 Apr 7]. Available from: http://www.ama-assn.org/ama/pub/news/news/2013/2013-06-18-new-ama-policies-annual-meeting.page .
- 2. Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet [Internet] doi: 10.1016/S0140-6736(14)60460-8. (0). Available from: http://www.sciencedirect.com/science/article/pii/S0140673614604608 . [ DOI ] [ PMC free article ] [ PubMed ]
- 3. Stevens GA, Singh GM, Lu Y, Danaei G, Lin JK, Finucane MM, et al. National, regional, and global trends in adult overweight and obesity prevalences. Popul Health Metr. 2012;10(1):22. doi: 10.1186/1478-7954-10-22. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. Kelly T, Yang W, Chen C-S, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes 2005. 2008 Sep;32(9):1431–7. doi: 10.1038/ijo.2008.102. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all Americans become overweight or obese? estimating the progression and cost of the US obesity epidemic. Obes Silver Spring Md. 2008 Oct;16(10):2323–30. doi: 10.1038/oby.2008.351. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Hu FB. Obesity epidemiology. Oxford University Press; Oxford; New York: 2008. p. 498. [ Google Scholar ]
- 7. Hu FB. Obesity and Mortality: Watch Your Waist, Not Just Your Weight. Arch Intern Med. 2007 May 14;167(9):875. doi: 10.1001/archinte.167.9.875. [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009 Oct 20;120(16):1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Alberti KGM, Zimmet P, Shaw J. The metabolic syndrome—a new worldwide definition. The Lancet. 366(9491):1059–62. doi: 10.1016/S0140-6736(05)67402-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. WHO Multicentre Growth Reference Study Group WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Oslo Nor 1992 Suppl. 2006 Apr;450:76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. De Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007 Sep;85(9):660–7. doi: 10.2471/BLT.07.043497. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 12. Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data. 2000 Jun 8;(314):1–27. [ PubMed ] [ Google Scholar ]
- 13. Barlow SE, the Expert Committee Expert Committee Recommendations Regarding the Prevention, Assessment, and Treatment of Child and Adolescent Overweight and Obesity: Summary Report. Pediatrics. 2007 Dec 1;120(Supplement 4):S164–92. doi: 10.1542/peds.2007-2329C. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Flegal KM, Wei R, Ogden CL, Freedman DS, Johnson CL, Curtin LR. Characterizing extreme values of body mass index-for-age by using the 2000 Centers for Disease Control and Prevention growth charts. Am J Clin Nutr. 2009 Nov;90(5):1314–20. doi: 10.3945/ajcn.2009.28335. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Grummer-Strawn LM, Reinold C, Krebs NF, Centers for Disease Control and Prevention (CDC) Use of World Health Organization and CDC growth charts for children aged 0-59 months in the United States. MMWR Recomm Rep Morb Mortal Wkly Rep Recomm Rep Cent Dis Control. 2010 Sep 10;59(RR-9):1–15. [ PubMed ] [ Google Scholar ]
- 16. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of Childhood and Adult Obesity in the United States, 2011-2012. JAMA. 2014 Feb 26;311(8):806. doi: 10.1001/jama.2014.732. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 17. Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960-1994. Int J Obes Relat Metab Disord J Int Assoc Study Obes. 1998 Jan;22(1):39–47. doi: 10.1038/sj.ijo.0800541. [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Von Ruesten A, Steffen A, Floegel A, van der ADL, Masala G, Tjønneland A, et al. Trend in obesity prevalence in European adult cohort populations during follow-up since 1996 and their predictions to 2015. PloS One. 2011;6(11):e27455. doi: 10.1371/journal.pone.0027455. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 19. Berghöfer A, Pischon T, Reinhold T, Apovian CM, Sharma AM, Willich SN. Obesity prevalence from a European perspective: a systematic review. BMC Public Health. 2008;8(1):200. doi: 10.1186/1471-2458-8-200. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. Skinner AC, Skelton JA. Prevalence and Trends in Obesity and Severe Obesity Among Children in the United States, 1999-2012. JAMA Pediatr [Internet] 2014 Apr 7; doi: 10.1001/jamapediatrics.2014.21. [cited 2014 Apr 8]; Available from: http://archpedi.jamanetwork.com/article.aspx?doi=10.1001/jamapediatrics.2014.21 . [ DOI ] [ PubMed ]
- 21. Wijnhoven TMA, van Raaij JMA, Spinelli A, Rito AI, Hovengen R, Kunesova M, et al. WHO European Childhood Obesity Surveillance Initiative 2008: weight, height and body mass index in 6–9-year-old children. Pediatr Obes. 2013;8(2):79–97. doi: 10.1111/j.2047-6310.2012.00090.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Barquera S, Campos-Nonato I, Hernández-Barrera L, Pedroza A, Rivera-Dommarco JA. [Prevalence of obesity in Mexican adults 2000-2012] Salud Pública México. 2013;55(Suppl 2):S151–60. [ PubMed ] [ Google Scholar ]
- 23. Liang Y-J, Xi B, Song A-Q, Liu J-X, Mi J. Trends in general and abdominal obesity among Chinese children and adolescents 1993-2009: General and abdominal obesity in Chinese children. Pediatr Obes. 2012 Oct;7(5):355–64. doi: 10.1111/j.2047-6310.2012.00066.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010 Nov;42(11):937–48. doi: 10.1038/ng.686. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 25. Hindorff LA, Sethupathy P, Junkins HA, Ramos EM, Mehta JP, Collins FS, et al. Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc Natl Acad Sci. 2009 Jun 9;106(23):9362–7. doi: 10.1073/pnas.0903103106. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet. 1962 Dec;14:353–62. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 27. Kilpeläinen TO, Qi L, Brage S, Sharp SJ, Sonestedt E, Demerath E, et al. Physical Activity Attenuates the Influence of FTO Variants on Obesity Risk: A Meta-Analysis of 218,166 Adults and 19,268 Children. In: Lewis C, editor. PLoS Med. 11. Vol. 8. Nov 1, 2011. p. e1001116. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 28. Rankinen T, Rice T, Teran-Garcia M, Rao DC, Bouchard C. FTO genotype is associated with exercise training-induced changes in body composition. Obes Silver Spring Md. 2010 Feb;18(2):322–6. doi: 10.1038/oby.2009.205. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Scott RA, Bailey MES, Moran CN, Wilson RH, Fuku N, Tanaka M, et al. FTO genotype and adiposity in children: physical activity levels influence the effect of the risk genotype in adolescent males. Eur J Hum Genet EJHG. 2010 Dec;18(12):1339–43. doi: 10.1038/ejhg.2010.131. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 30. Ravelli AC, van der Meulen JH, Osmond C, Barker DJ, Bleker OP. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr. 1999 Nov 1;70(5):811–6. doi: 10.1093/ajcn/70.5.811. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Drake AJ, Reynolds RM. Impact of maternal obesity on offspring obesity and cardiometabolic disease risk. Reproduction. 2010 Sep 1;140(3):387–98. doi: 10.1530/REP-10-0077. [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Gaillard R, Steegers EAP, Duijts L, Felix JF, Hofman A, Franco OH, et al. Childhood Cardiometabolic Outcomes of Maternal Obesity During Pregnancy: The Generation R Study. Hypertension. 2014 Apr 1;63(4):683–91. doi: 10.1161/HYPERTENSIONAHA.113.02671. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Bammann K, Peplies J, De Henauw S, Hunsberger M, Molnar D, Moreno LA, et al. Early Life Course Risk Factors for Childhood Obesity: The IDEFICS Case-Control Study. In: Bruce A, editor. PLoS ONE. 2. Vol. 9. Feb 13, 2014. p. e86914. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 34. Northstone K, Golding J, Davey Smith G, Miller LL, Pembrey M. Prepubertal start of father’s smoking and increased body fat in his sons: further characterisation of paternal transgenerational responses. Eur J Hum Genet EJHG. 2014 Apr 2; doi: 10.1038/ejhg.2014.31. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Janesick AS, Shioda T, Blumberg B. Transgenerational inheritance of prenatal obesogen exposure. Mol Cell Endocrinol. 2014 Sep 15; doi: 10.1016/j.mce.2014.09.002. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 36. De Cock M, van de Bor M. Obesogenic effects of endocrine disruptors, what do we know from animal and human studies? Environ Int. 2014 Sep;70:15–24. doi: 10.1016/j.envint.2014.04.022. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Dabelea D, Harrod CS. Role of developmental overnutrition in pediatric obesity and type 2 diabetes. Nutr Rev. 2013 Oct;71:S62–7. doi: 10.1111/nure.12061. [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA J Am Med Assoc. 2004 Mar. 10;291(10):1238–45. doi: 10.1001/jama.291.10.1238. [ DOI ] [ PubMed ] [ Google Scholar ]
- 39. Hill JO, Wyatt HR, Peters JC. Energy Balance and Obesity. Circulation. 2012 Jul 3;126(1):126–32. doi: 10.1161/CIRCULATIONAHA.111.087213. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 40. Wadden TA, Webb VL, Moran CH, Bailer BA. Lifestyle Modification for Obesity: New Developments in Diet, Physical Activity, and Behavior Therapy. Circulation. 2012 Mar 6;125(9):1157–70. doi: 10.1161/CIRCULATIONAHA.111.039453. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 41. Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, et al. Weight Loss with a Low-Carbohydrate, Mediterranean, or Low-Fat Diet. N Engl J Med. 2008 Jul 17;359(3):229–41. doi: 10.1056/NEJMoa0708681. [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Gardner CD. Comparison of the Atkins, Zone, Ornish, and LEARN Diets for Change in Weight and Related Risk Factors Among Overweight Premenopausal Women<SUBTITLE>The A TO Z Weight Loss Study: A Randomized Trial</SUBTITLE>. JAMA. 2007 Mar 7;297(9):969. doi: 10.1001/jama.297.9.969. [ DOI ] [ PubMed ] [ Google Scholar ]
- 43. Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009 Feb 26;360(9):859–73. doi: 10.1056/NEJMoa0804748. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 44. Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in Diet and Lifestyle and Long-Term Weight Gain in Women and Men. N Engl J Med. 2011 Jun 22;364(25):2392–404. doi: 10.1056/NEJMoa1014296. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 45. Malik VS, Popkin BM, Bray GA, Despres J-P, Hu FB. Sugar-Sweetened Beverages, Obesity, Type 2 Diabetes Mellitus, and Cardiovascular Disease Risk. Circulation. 2010 Mar 23;121(11):1356–64. doi: 10.1161/CIRCULATIONAHA.109.876185. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 46. Malik VS, Pan A, Willett WC, Hu FB. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am J Clin Nutr. 2013 Oct 1;98(4):1084–102. doi: 10.3945/ajcn.113.058362. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 47. Hu FB. Resolved: there is sufficient scientific evidence that decreasing sugar-sweetened beverage consumption will reduce the prevalence of obesity and obesity-related diseases. Obes Rev. 2013 Aug 1;14(8):606–19. doi: 10.1111/obr.12040. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 48. Gallagher J. Mexico restricts soft drink TV ads to fight obesity [Internet] BBC News. 2014 [cited 2014 Jul 16]. Available from: http://www.bbc.com/news/world-latin-america-28325105 .
- 49. New York City bans supersize sodas [Internet] BBC News. 2012 [cited 2014 Jul 21]. Available from: http://www.bbc.com/news/world-us-canada-19593012 .
- 50. 2008 Physical Activity Guidelines for Americans [Internet] [cited 2014 Apr 21]. Available from: http://www.health.gov/paguidelines/guidelines/
- 51. Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK, et al. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009 Feb;41(2):459–71. doi: 10.1249/MSS.0b013e3181949333. [ DOI ] [ PubMed ] [ Google Scholar ]
- 52. Patel SR, Hu FB. Short Sleep Duration and Weight Gain: A Systematic Review. Obesity. 2008 Mar;16(3):643–53. doi: 10.1038/oby.2007.118. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 53. Watanabe M, Kikuchi H, Tanaka K, Takahashi M. Association of short sleep duration with weight gain and obesity at 1-year follow-up: a large-scale prospective study. Sleep. 2010 Feb;33(2):161–7. doi: 10.1093/sleep/33.2.161. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 54. Kobayashi D, Takahashi O, Deshpande GA, Shimbo T, Fukui T. Association between weight gain, obesity, and sleep duration: a large-scale 3-year cohort study. Sleep Breath Schlaf Atm. 2012 Sep;16(3):829–33. doi: 10.1007/s11325-011-0583-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 55. Lyytikäinen P, Rahkonen O, Lahelma E, Lallukka T. Association of sleep duration with weight and weight gain: a prospective follow-up study. J Sleep Res. 2011 Jun;20(2):298–302. doi: 10.1111/j.1365-2869.2010.00903.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 56. Hu FB. Television Watching and Other Sedentary Behaviors in Relation to Risk of Obesity and Type 2 Diabetes Mellitus in Women. JAMA. 2003 Apr 9;289(14):1785. doi: 10.1001/jama.289.14.1785. [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Boone JE, Gordon-Larsen P, Adair LS, Popkin BM. Screen time and physical activity during adolescence: longitudinal effects on obesity in young adulthood. Int J Behav Nutr Phys Act. 2007;4(1):26. doi: 10.1186/1479-5868-4-26. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 58. Martínez-González MA, Martínez JA, Hu FB, Gibney MJ, Kearney J. Physical inactivity, sedentary lifestyle and obesity in the European Union. Int J Obes Relat Metab Disord J Int Assoc Study Obes. 1999 Nov;23(11):1192–201. doi: 10.1038/sj.ijo.0801049. [ DOI ] [ PubMed ] [ Google Scholar ]
- 59. Levine JA. Poverty and Obesity in the U.S. Diabetes. 2011 Nov 1;60(11):2667–8. doi: 10.2337/db11-1118. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 60. Koh KA, Hoy JS, O’Connell JJ, Montgomery P. The hunger-obesity paradox: obesity in the homeless. J Urban Health Bull N Y Acad Med. 2012 Dec;89(6):952–64. doi: 10.1007/s11524-012-9708-4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 61. Tsai J, Rosenheck RA. Obesity among chronically homeless adults: is it a problem? Public Health Rep Wash DC 1974. 2013 Feb;128(1):29–36. doi: 10.1177/003335491312800105. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 62. Devaux M, Sassi F. Social inequalities in obesity and overweight in 11 OECD countries. Eur J Public Health. 2013 Jun 1;23(3):464–9. doi: 10.1093/eurpub/ckr058. [ DOI ] [ PubMed ] [ Google Scholar ]
- 63. Aitsi-Selmi A, Bell R, Shipley MJ, Marmot MG. Education modifies the association of wealth with obesity in women in middle-income but not low-income countries: an interaction study using seven national datasets, 2005-2010. PloS One. 2014;9(3):e90403. doi: 10.1371/journal.pone.0090403. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 64. Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol. 2013 Jan;9(1):13–27. doi: 10.1038/nrendo.2012.199. [ DOI ] [ PubMed ] [ Google Scholar ]
- 65. Regnier SM, Sargis RM. Adipocytes under assault: environmental disruption of adipose physiology. Biochim Biophys Acta. 2014 Mar;1842(3):520–33. doi: 10.1016/j.bbadis.2013.05.028. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 66. Papas MA, Alberg AJ, Ewing R, Helzlsouer KJ, Gary TL, Klassen AC. The built environment and obesity. Epidemiol Rev. 2007;29:129–43. doi: 10.1093/epirev/mxm009. [ DOI ] [ PubMed ] [ Google Scholar ]
- 67. Larson NI, Story MT, Nelson MC. Neighborhood environments: disparities in access to healthy foods in the U.S. Am J Prev Med. 2009 Jan;36(1):74–81. doi: 10.1016/j.amepre.2008.09.025. [ DOI ] [ PubMed ] [ Google Scholar ]
- 68. Holsten JE. Obesity and the community food environment: a systematic review. Public Health Nutr. 2009 Mar;12(3):397–405. doi: 10.1017/S1368980008002267. [ DOI ] [ PubMed ] [ Google Scholar ]
- 69. McCormack GR, Shiell A. In search of causality: a systematic review of the relationship between the built environment and physical activity among adults. Int J Behav Nutr Phys Act. 2011;8:125. doi: 10.1186/1479-5868-8-125. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 70. Morland KB, Evenson KR. Obesity prevalence and the local food environment. Health Place. 2009 Jun;15(2):491–5. doi: 10.1016/j.healthplace.2008.09.004. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 71. Sallis JF, Floyd MF, Rodríguez DA, Saelens BE. Role of built environments in physical activity, obesity, and cardiovascular disease. Circulation. 2012 Feb 7;125(5):729–37. doi: 10.1161/CIRCULATIONAHA.110.969022. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 72. Durand CP, Andalib M, Dunton GF, Wolch J, Pentz MA. A systematic review of built environment factors related to physical activity and obesity risk: implications for smart growth urban planning. Obes Rev Off J Int Assoc Study Obes. 2011 May;12(5):e173–82. doi: 10.1111/j.1467-789X.2010.00826.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 73. Berry TR, Spence JC, Blanchard CM, Cutumisu N, Edwards J, Selfridge G. A longitudinal and cross-sectional examination of the relationship between reasons for choosing a neighbourhood, physical activity and body mass index. Int J Behav Nutr Phys Act. 2010;7:57. doi: 10.1186/1479-5868-7-57. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 74. Boone-Heinonen J, Diez-Roux AV, Goff DC, Loria CM, Kiefe CI, Popkin BM, et al. The neighborhood energy balance equation: does neighborhood food retail environment + physical activity environment = obesity? The CARDIA study. PloS One. 2013;8(12):e85141. doi: 10.1371/journal.pone.0085141. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 75. Auchincloss AH, Mujahid MS, Shen M, Michos ED, Whitt-Glover MC, Diez Roux AV. Neighborhood health-promoting resources and obesity risk (the multi-ethnic study of atherosclerosis) Obes Silver Spring Md. 2013 Mar;21(3):621–8. doi: 10.1038/oby.2012.91. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 76. McAllister EJ, Dhurandhar NV, Keith SW, Aronne LJ, Barger J, Baskin M, et al. Ten Putative Contributors to the Obesity Epidemic. Crit Rev Food Sci Nutr. 2009 Dec 10;49(10):868–913. doi: 10.1080/10408390903372599. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 77. Parra-Rojas I, Del Moral-Hernández O, Salgado-Bernabé AB, Guzmán-Guzmán IP, Salgado-Goytia L, Muñoz-Valle JF. Adenovirus-36 seropositivity and its relation with obesity and metabolic profile in children. Int J Endocrinol. 2013;2013:463194. doi: 10.1155/2013/463194. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 78. Atkinson RL, Dhurandhar NV, Allison DB, Bowen RL, Israel BA, Albu JB, et al. Human adenovirus-36 is associated with increased body weight and paradoxical reduction of serum lipids. Int J Obes 2005. 2005 Mar;29(3):281–6. doi: 10.1038/sj.ijo.0802830. [ DOI ] [ PubMed ] [ Google Scholar ]
- 79. Almgren M, Atkinson R, He J, Hilding A, Hagman E, Wolk A, et al. Adenovirus-36 is associated with obesity in children and adults in Sweden as determined by rapid ELISA. PloS One. 2012;7(7):e41652. doi: 10.1371/journal.pone.0041652. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 80. Clarke SF, Murphy EF, Nilaweera K, Ross PR, Shanahan F, O’Toole PW, et al. The gut microbiota and its relationship to diet and obesity: new insights. Gut Microbes. 2012 Jun;3(3):186–202. doi: 10.4161/gmic.20168. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 81. Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: Human gut microbes associated with obesity. Nature. 2006 Dec 21;444(7122):1022–3. doi: 10.1038/4441022a. [ DOI ] [ PubMed ] [ Google Scholar ]
- 82. David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2013 Dec 11;505(7484):559–63. doi: 10.1038/nature12820. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 83. Wu GD, Chen J, Hoffmann C, Bittinger K, Chen Y-Y, Keilbaugh SA, et al. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science. 2011 Oct 7;334(6052):105–8. doi: 10.1126/science.1208344. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 84. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006 Dec 21;444(7122):1027–131. doi: 10.1038/nature05414. [ DOI ] [ PubMed ] [ Google Scholar ]
- 85. Shen J, Obin MS, Zhao L. The gut microbiota, obesity and insulin resistance. Obesity. 2013 Feb;34(1):39–58. doi: 10.1016/j.mam.2012.11.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 86. Christakis NA, Fowler JH. The Spread of Obesity in a Large Social Network over 32 Years. N Engl J Med. 2007 Jul 26;357(4):370–9. doi: 10.1056/NEJMsa066082. [ DOI ] [ PubMed ] [ Google Scholar ]
- 87. Leroux JS, Moore S, Dubé L. Beyond the “I” in the Obesity Epidemic: A Review of Social Relational and Network Interventions on Obesity. J Obes. 2013;2013:1–10. doi: 10.1155/2013/348249. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 88. Peeters A, Barendregt JJ, Willekens F, Mackenbach JP, Al Mamun A, Bonneux L, et al. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med. 2003 Jan 7;138(1):24–32. doi: 10.7326/0003-4819-138-1-200301070-00008. [ DOI ] [ PubMed ] [ Google Scholar ]
- 89. Prospective Studies Collaboration. Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009 Mar 28;373(9669):1083–96. doi: 10.1016/S0140-6736(09)60318-4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 90. Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, et al. Body-Mass Index and Mortality among 1.46 Million White Adults. N Engl J Med. 2010 Dec 1;363(23):2211–9. doi: 10.1056/NEJMoa1000367. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 91. Chen Y, Copeland WK, Vedanthan R, Grant E, Lee JE, Gu D, et al. Association between body mass index and cardiovascular disease mortality in east Asians and south Asians: pooled analysis of prospective data from the Asia Cohort Consortium. BMJ. 2013 Oct 1;347:f5446–f5446. doi: 10.1136/bmj.f5446. oct01 1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 92. Tsai SP, Donnelly RP, Wendt JK. Obesity and mortality in a prospective study of a middle-aged industrial population. J Occup Environ Med Am Coll Occup Environ Med. 2006 Jan;48(1):22–7. doi: 10.1097/01.jom.0000184866.49000.e5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 93. Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006 Aug 24;355(8):763–78. doi: 10.1056/NEJMoa055643. [ DOI ] [ PubMed ] [ Google Scholar ]
- 94. Adams KF, Leitzmann MF, Ballard-Barbash R, Albanes D, Harris TB, Hollenbeck A, et al. Body Mass and Weight Change in Adults in Relation to Mortality Risk. Am J Epidemiol. 2014 Jan 15;179(2):135–44. doi: 10.1093/aje/kwt254. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 95. Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW. Body-Mass Index and Mortality in a Prospective Cohort of U.S. Adults. N Engl J Med. 1999 Oct 7;341(15):1097–105. doi: 10.1056/NEJM199910073411501. [ DOI ] [ PubMed ] [ Google Scholar ]
- 96. Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, Obesity, and Mortality from Cancer in a Prospectively Studied Cohort of U.S. Adults. N Engl J Med. 2003 Apr 24;348(17):1625–38. doi: 10.1056/NEJMoa021423. [ DOI ] [ PubMed ] [ Google Scholar ]
- 97. Park Y, Wang S, Kitahara CM, Moore SC, Berrington de Gonzalez A, Bernstein L, et al. Body Mass Index and Risk of Death in Asian Americans. Am J Public Health. 2014 Mar;104(3):520–5. doi: 10.2105/AJPH.2013.301573. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 98. American Diabetes Association Standards of Medical Care in Diabetes—2012. Diabetes Care. 2012 Jan 1;35(Supplement 1):S11–63. doi: 10.2337/dc12-s011. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 99. Abdullah A, Peeters A, de Courten M, Stoelwinder J. The magnitude of association between overweight and obesity and the risk of diabetes: A meta-analysis of prospective cohort studies. Diabetes Res Clin Pract. 2010 Sep;89(3):309–19. doi: 10.1016/j.diabres.2010.04.012. [ DOI ] [ PubMed ] [ Google Scholar ]
- 100. Reilly JJ, Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int J Obes. 2011 Jul;35(7):891–8. doi: 10.1038/ijo.2010.222. [ DOI ] [ PubMed ] [ Google Scholar ]
- 101. Kodama S, Horikawa C, Fujihara K, Yoshizawa S, Yachi Y, Tanaka S, et al. Quantitative relationship between body weight gain in adulthood and incident type 2 diabetes: a meta-analysis: Body weight gain and type 2 diabetes. Obes Rev. 2014 Mar;15(3):202–14. doi: 10.1111/obr.12129. [ DOI ] [ PubMed ] [ Google Scholar ]
- 102. Narayan KMV, Boyle JP, Thompson TJ, Gregg EW, Williamson DF. Effect of BMI on Lifetime Risk for Diabetes in the U.S. Diabetes Care. 2007 Jun 1;30(6):1562–6. doi: 10.2337/dc06-2544. [ DOI ] [ PubMed ] [ Google Scholar ]
- 103. National Diabetes Information Clearinghouse, US Department of Health and Human Services Diabetes Fact Sheet [Internet] [cited 2014 Apr 24]. Available from: http://diabetes.niddk.nih.gov/dm/pubs/overview/
- 104. Bell JA, Kivimaki M, Hamer M. Metabolically healthy obesity and risk of incident type 2 diabetes: a meta-analysis of prospective cohort studies. Obes Rev. 2014 doi: 10.1111/obr.12157. n/a – n/a. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 105. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet. 2012 Dec 15;380(9859):2095–128. doi: 10.1016/S0140-6736(12)61728-0. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 106. Flint AJ, Hu FB, Glynn RJ, Caspard H, Manson JE, Willett WC, et al. Excess Weight and the Risk of Incident Coronary Heart Disease Among Men and Women. Obesity. 2010 Feb;18(2):377–83. doi: 10.1038/oby.2009.223. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 107. Kramer CK, Zinman B, Retnakaran R. Are Metabolically Healthy Overweight and Obesity Benign Conditions?: A Systematic Review and Meta-analysis. Ann Intern Med. 2013 Dec 3;159(11):758. doi: 10.7326/0003-4819-159-11-201312030-00008. [ DOI ] [ PubMed ] [ Google Scholar ]
- 108. Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration (BMI Mediated Effects) Lu Y, Hajifathalian K, Ezzati M, Woodward M, Rimm EB, et al. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1·8 million participants. Lancet. 2014 Mar 15;383(9921):970–83. doi: 10.1016/S0140-6736(13)61836-X. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 109. Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, et al. Childhood Adiposity, Adult Adiposity, and Cardiovascular Risk Factors. N Engl J Med. 2011 Nov 16;365(20):1876–85. doi: 10.1056/NEJMoa1010112. [ DOI ] [ PubMed ] [ Google Scholar ]
- 110. Polednak AP. Estimating the number of U.S. incident cancers attributable to obesity and the impact on temporal trends in incidence rates for obesity-related cancers. Cancer Detect Prev. 2008 Jan;32(3):190–9. doi: 10.1016/j.cdp.2008.08.004. [ DOI ] [ PubMed ] [ Google Scholar ]
- 111. Vainio H, Bianchini F. Weight control and physical activity. IARC Press; Lyon: 2002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 112. Larsson SC, Wolk A. Obesity and the risk of gallbladder cancer: a meta-analysis. Br J Cancer [Internet] 2007 Mar 20; doi: 10.1038/sj.bjc.6603703. [cited 2014 Apr 24]; Available from: http://www.nature.com/doifinder/10.1038/sj.bjc.6603703 . [ DOI ] [ PMC free article ] [ PubMed ]
- 113. Larsson SC, Wolk A. Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br J Cancer. 2007 Oct 8;97(7):1005–8. doi: 10.1038/sj.bjc.6603932. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 114. Olsen CM, Green AC, Whiteman DC, Sadeghi S, Kolahdooz F, Webb PM. Obesity and the risk of epithelial ovarian cancer: a systematic review and meta-analysis. Eur J Cancer Oxf Engl 1990. 2007 Mar;43(4):690–709. doi: 10.1016/j.ejca.2006.11.010. [ DOI ] [ PubMed ] [ Google Scholar ]
- 115. Discacciati A, Orsini N, Wolk A. Body mass index and incidence of localized and advanced prostate cancer--a dose-response meta-analysis of prospective studies. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2012 Jul;23(7):1665–71. doi: 10.1093/annonc/mdr603. [ DOI ] [ PubMed ] [ Google Scholar ]
- 116. Larsson SC, Wolk A. Overweight and obesity and incidence of leukemia: a meta-analysis of cohort studies. Int J Cancer J Int Cancer. 2008 Mar 15;122(6):1418–21. doi: 10.1002/ijc.23176. [ DOI ] [ PubMed ] [ Google Scholar ]
- 117. Glance LG, Li Y, Osler TM, Mukamel DB, Dick AW. Impact of Obesity on Mortality and Complications in Trauma Patients. Ann Surg. 2014 Mar;259(3):576–81. doi: 10.1097/SLA.0000000000000330. [ DOI ] [ PubMed ] [ Google Scholar ]
- 118. Liu T, Chen J, Bai X, Zheng G, Gao W. The effect of obesity on outcomes in trauma patients: A meta-analysis. Injury. 2013 Sep;44(9):1145–52. doi: 10.1016/j.injury.2012.10.038. [ DOI ] [ PubMed ] [ Google Scholar ]
- 119. Huttunen R, Syrjänen J. Obesity and the risk and outcome of infection. Int J Obes. 2013 Mar;37(3):333–40. doi: 10.1038/ijo.2012.62. [ DOI ] [ PubMed ] [ Google Scholar ]
- 120. Young KM, Gray CM, Bekker L-G. Is Obesity a Risk Factor for Vaccine Non-Responsiveness? Ahuja SK, editor. PLoS ONE. 2013 Dec 11;8(12):e82779. doi: 10.1371/journal.pone.0082779. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 121. Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, et al. Brain structure and obesity. Hum Brain Mapp. 2009 doi: 10.1002/hbm.20870. NA – NA. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 122. Reinert KRS, Po’e EK, Barkin SL. The Relationship between Executive Function and Obesity in Children and Adolescents: A Systematic Literature Review. J Obes. 2013;2013:1–10. doi: 10.1155/2013/820956. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 123. Anstey KJ, Cherbuin N, Budge M, Young J. Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes Rev Off J Int Assoc Study Obes. 2011 May 12;(5):e426–37. doi: 10.1111/j.1467-789X.2010.00825.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 124. Lindholm V, Lahti J, Rahkonen O, Lahelma E, Lallukka T. Joint association of physical activity and body weight with subsequent physical and mental functioning: a follow-up study. BMC Public Health. 2013;13(1):197. doi: 10.1186/1471-2458-13-197. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 125. Cawley J, Meyerhoefer C. The medical care costs of obesity: An instrumental variables approach. J Health Econ. 2012 Jan;31(1):219–30. doi: 10.1016/j.jhealeco.2011.10.003. [ DOI ] [ PubMed ] [ Google Scholar ]
- 126. Mason RJ, Moroney JR, Berne TV. The cost of obesity for nonbariatric inpatient operative procedures in the United States: national cost estimates obese versus nonobese patients. Ann Surg. 2013 Oct;258(4):541–51. doi: 10.1097/SLA.0b013e3182a500ce. discussion 551–3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 127. Kleinman N, Abouzaid S, Andersen L, Wang Z, Powers A. Cohort Analysis Assessing Medical and Nonmedical Cost Associated With Obesity in the Workplace: J Occup Environ Med. 2014 Feb;56(2):161–70. doi: 10.1097/JOM.0000000000000099. [ DOI ] [ PubMed ] [ Google Scholar ]
- 128. Finkelstein EA, Graham WCK, Malhotra R. Lifetime Direct Medical Costs of Childhood Obesity. PEDIATRICS [Internet] 2014 Apr 7; doi: 10.1542/peds.2014-0063. [cited 2014 Apr 22]; Available from: http://pediatrics.aappublications.org/cgi/doi/10.1542/peds.2014-0063 . [ DOI ] [ PubMed ]
- 129. Müller-Riemenschneider F, Reinhold T, Berghöfer A, Willich SN. Health-economic burden of obesity in Europe. Eur J Epidemiol. 2008 Aug;23(8):499–509. doi: 10.1007/s10654-008-9239-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 130. Von Lengerke T, Krauth C. Economic costs of adult obesity: A review of recent European studies with a focus on subgroup-specific costs. Maturitas. 2011 Jul;69(3):220–9. doi: 10.1016/j.maturitas.2011.04.005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 131. Bahia L, Coutinho ES, Barufaldi L, de Azevedo Abreu G, Malhão T, Ribeiro de Souza C, et al. The costs of overweight and obesity-related diseases in the Brazilian public health system: cross-sectional study. BMC Public Health. 2012;12(1):440. doi: 10.1186/1471-2458-12-440. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 132. Rtveladze K, Marsh T, Webber L, Kilpi F, Levy D, Conde W, et al. Health and economic burden of obesity in Brazil. PloS One. 2013;8(7):e68785. doi: 10.1371/journal.pone.0068785. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 133. Rtveladze K, Marsh T, Barquera S, Sanchez Romero LM, Levy D, Melendez G, et al. Obesity prevalence in Mexico: impact on health and economic burden. Public Health Nutr. 2014 Jan;17(01):233–9. doi: 10.1017/S1368980013000086. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 134. Marketdata Enterprises, Inc. The U.S. Weight Loss Market: 2014 Status Report & Market Research [Internet] 2014 [cited 2014 Apr 23]. Available from: http://www.marketresearch.com/Marketdata-Enterprises-Inc-v416/Weight-Loss-Status-Forecast-8016030/
- 135. National Institutes of Health, National Heart, Lung, and Blood Institute . Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults. [Internet] U.S. Department of Health and Human Services; Bethesda, MD: Oct, 1998. Obesity Education Initiative: Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. [cited 2014 Apr 1]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK2003/ [ Google Scholar ]
- 136. Huang TT, Drewnosksi A, Kumanyika S, Glass TA. A systems-oriented multilevel framework for addressing obesity in the 21st century. Prev Chronic Dis. 2009 Jul;6(3):A82. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 137. Cornelis MC, Hu FB. Systems Epidemiology: A New Direction in Nutrition and Metabolic Disease Research. Curr Nutr Rep. 2013 Dec;2(4) doi: 10.1007/s13668-013-0052-4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 138. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health . The Health Consequences of Smoking —50 Years of Progress: A Report of the Surgeon General. [Internet] U.S. Department of Health and Human Services; Atlanta, GA: 2014. [cited 2014 Apr 1]. Available from: http://www.surgeongeneral.gov/library/reports/50-years-of-progress/ [ Google Scholar ]
- 139. World Health Organization . Obesity: preventing and managing the global epidemic: report of a WHO consultation. World Health Organization; Geneva: 2000. p. 253. [ PubMed ] [ Google Scholar ]
- 140. Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000 May 6;320(7244):1240. doi: 10.1136/bmj.320.7244.1240. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 141. Fryar CD, Carroll MD, Ogden CL. NCHS Health E Stat - Overweight, Obesity, and Extreme Obesity Among Adults: United States, Trends 1960-1962 Through 2009-2010 [Internet] [cited 2014 Feb 26]. Available from: http://www.cdc.gov/nchs/data/hestat/obesity_adult_09_10/obesity_adult_09_10.htm .
- View on publisher site
- PDF (645.4 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 23 September 2021
The genetics of obesity: from discovery to biology
- Ruth J. F. Loos ORCID: orcid.org/0000-0002-8532-5087 1 , 2 , 3 , 4 &
- Giles S. H. Yeo ORCID: orcid.org/0000-0001-8823-3615 5
Nature Reviews Genetics volume 23 , pages 120–133 ( 2022 ) Cite this article
172k Accesses
513 Citations
493 Altmetric
Metrics details
- Disease genetics
- Endocrine system and metabolic diseases
- Genetic association study
- Genetic variation
The prevalence of obesity has tripled over the past four decades, imposing an enormous burden on people’s health. Polygenic (or common) obesity and rare, severe, early-onset monogenic obesity are often polarized as distinct diseases. However, gene discovery studies for both forms of obesity show that they have shared genetic and biological underpinnings, pointing to a key role for the brain in the control of body weight. Genome-wide association studies (GWAS) with increasing sample sizes and advances in sequencing technology are the main drivers behind a recent flurry of new discoveries. However, it is the post-GWAS, cross-disciplinary collaborations, which combine new omics technologies and analytical approaches, that have started to facilitate translation of genetic loci into meaningful biology and new avenues for treatment.
Similar content being viewed by others
A phenome-wide comparative analysis of genetic discordance between obesity and type 2 diabetes

Multivariate genomic analysis of 5 million people elucidates the genetic architecture of shared components of the metabolic syndrome

Obesity: exploring its connection to brain function through genetic and genomic perspectives
Introduction.
Obesity is associated with premature mortality and is a serious public health threat that accounts for a large proportion of the worldwide non-communicable disease burden, including type 2 diabetes, cardiovascular disease, hypertension and certain cancers 1 , 2 . Mechanical issues resulting from substantially increased weight, such as osteoarthritis and sleep apnoea, also affect people’s quality of life 3 . The impact of obesity on communicable disease, in particular viral infection 4 , has recently been highlighted by the discovery that individuals with obesity are at increased risk of hospitalization and severe illness from COVID-19 (refs 5 , 6 , 7 ).
On the basis of the latest data from the NCD Risk Factor Collaboration, in 2016 almost 2 billion adults (39% of the world’s adult population) were estimated to be overweight (defined by a body mass index (BMI) of ≥25 kg m − 2 ), 671 million (12% of the world’s adult population) of whom had obesity (BMI ≥30 kg m − 2 ) — a tripling in the prevalence of obesity since 1975 (ref. 8 ) (Fig. 1 ). Although the rate of increase in obesity seems to be declining in most high-income countries, it continues to rise in many low-income and middle-income countries and prevalence remains high globally 8 . If current trends continue, it is expected that 1 billion adults (nearly 20% of the world population) will have obesity by 2025. Particularly alarming is the global rise in obesity among children and adolescents; more than 7% had obesity in 2016 compared with less than 1% in 1975 (ref. 8 ).
The prevalence of obesity has risen steadily over the past four decades in children, adolescents (not shown) and adults worldwide. a | Prevalence of obesity (body mass index (BMI) ≥30 kg m −2 ) in women and men ≥20 years of age, from 1975 to 2016. b | Prevalence of obesity (weight ≥2 s.d. above the median of the WHO growth reference) in 5-year-old girls and boys from 1975 to 2016. Geographical regions are represented by different colours. Graphs are reproduced from the NCD Risk Factor Collaboration (NCD RisC) website and are generated from data published in ref. 8 .
Although changes in the environment have undoubtedly driven the rapid increase in prevalence, obesity results from an interaction between environmental and innate biological factors. Crucially, there is a strong genetic component underlying the large interindividual variation in body weight that determines people’s response to this ‘obesogenic’ environment . Twin, family and adoption studies have estimated the heritability of obesity to be between 40% and 70% 9 , 10 . As a consequence, genetic approaches can be leveraged to characterize the underlying physiological and molecular mechanisms that control body weight.
Classically, we have considered obesity in two broad categories (Fig. 2 ): so-called monogenic obesity , which is inherited in a Mendelian pattern, is typically rare, early-onset and severe and involves either small or large chromosomal deletions or single-gene defects; and polygenic obesity (also known as common obesity), which is the result of hundreds of polymorphisms that each have a small effect. Polygenic obesity follows a pattern of heritability that is similar to other complex traits and diseases. Although often considered to be two distinct forms, gene discovery studies of monogenic and polygenic obesity have converged on what seems to be broadly similar underlying biology. Specifically, the central nervous system (CNS) and neuronal pathways that control the hedonic aspects of food intake have emerged as the major drivers of body weight for both monogenic and polygenic obesity. Furthermore, early evidence shows that the expression of mutations causing monogenic obesity may — at least in part — be influenced by the individual’s polygenic susceptibility to obesity 11 .
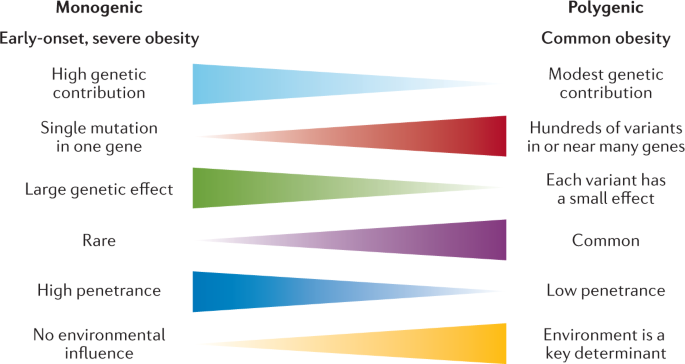
Key features of monogenic and polygenic forms of obesity .
In this Review, we summarize more than 20 years of genetic studies that have characterized the molecules and mechanisms that control body weight, specifically focusing on overall obesity and adiposity, rather than fat distribution or central adiposity. Although most of the current insights into the underlying biology have been derived from monogenic forms of obesity, recent years have witnessed several successful variant-to-function translations for polygenic forms of obesity. We also explore how the ubiquity of whole-exome sequencing (WES) and genome sequencing has begun to blur the line that used to demarcate the monogenic causes of obesity from common polygenic obesity. Syndromic forms of obesity, such as Bardet–Biedl, Prader–Willi, among many others 12 , are not reviewed here. Although obesity is often a dominant feature of these syndromes, the underlying genetic defects are often chromosomal abnormalities and typically encompass multiple genes, making it difficult to decipher the precise mechanisms directly related to body-weight regulation. Finally, as we enter the post-genomic era, we consider the prospects of genotype-informed treatments and the possibility of leveraging genetics to predict and hence prevent obesity.
Gene discovery approaches
The approaches used to identify genes linked to obesity depend on the form of obesity and genotyping technology available at the time. Early gene discovery studies for monogenic forms of obesity had a case-focused design: patients with severe obesity, together with their affected and unaffected family members, were examined for potential gene-disrupting causal mutations via Sanger sequencing. By contrast, genetic variation associated with common forms of obesity have been identified in large-scale population studies, either using a case–control design or continuous traits such as BMI. Gene discovery for both forms of obesity was initially hypothesis driven; that is, restricted to a set of candidate genes that evidence suggests have a role in body-weight regulation. Over the past two decades, however, advances in high-throughput genome-wide genotyping and sequencing technologies, combined with a detailed knowledge of the human genetic architecture, have enabled the interrogation of genetic variants across the whole genome for their role in body-weight regulation using a hypothesis-generating approach.
Gene discovery for monogenic obesity
Many of the candidate genes and pathways linked to body-weight regulation were initially identified in mice, such as the obese ( ob ) 13 and diabetes ( db ) 14 mouse lines, in which severe hyperphagia and obesity spontaneously emerged. Using reverse genetics , the ob gene was shown to encode leptin, a hormone produced from fat, and it was demonstrated that leptin deficiency resulting from a mutation in the ob gene caused the severe obesity seen in the ob/ob mouse 15 (Fig. 3 ). Shortly after the cloning of ob , the db gene was cloned and identified as encoding the leptin receptor (LEPR) 16 . Reverse genetics was also used to reveal that the complex obesity phenotype of Agouti ‘lethal yellow’ mice is caused by a rearrangement in the promoter sequence of the agouti gene that results in ectopic and constitutive expression of the agouti peptide 17 , 18 , which antagonizes the melanocortin 1 and 4 receptors (MC1R and MC4R) 19 , 20 . This finding linked the melanocortin pathway to body-weight regulation, thereby unveiling a whole raft of new candidate genes for obesity.
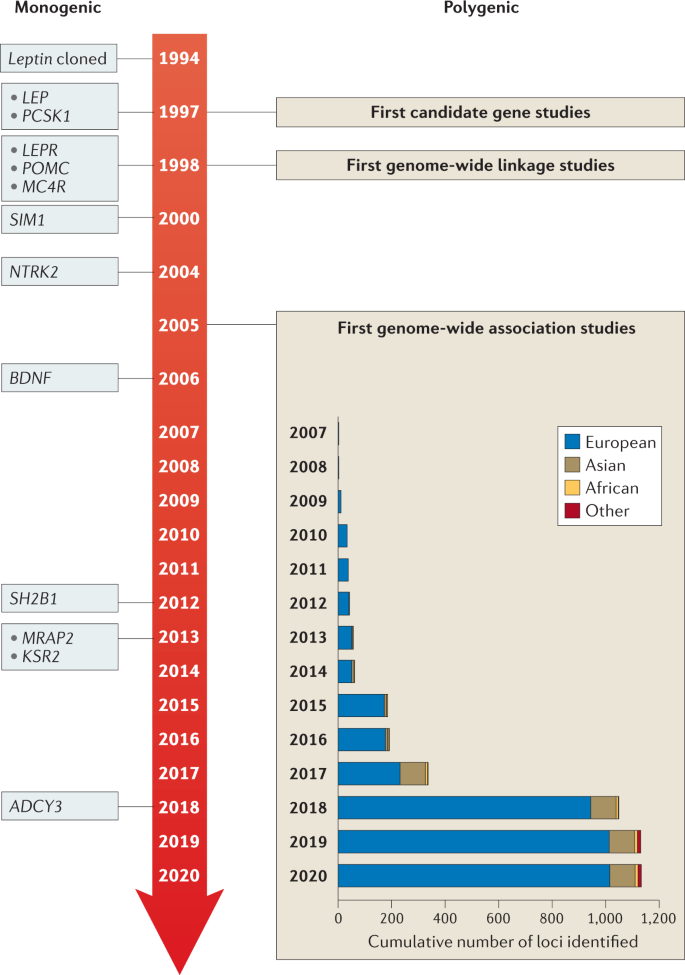
Genes identified for monogenic obesity in a given year are shown on the left. Discoveries made for polygenic obesity are shown on the right, including a cumulative count of newly discovered loci per year and by ancestry. Although candidate gene and genome-wide linkage studies became available in the late 1990s, findings were limited, and these study designs are not as frequently used as genome-wide association studies.
Once the genes for leptin and its receptor were identified, they became candidate genes for human obesity, and in 1997 the first humans with congenital leptin deficiency were identified 21 . This discovery was rapidly followed by the report of humans with mutations in the gene encoding the leptin receptor ( LEPR ) 22 , as well as in genes encoding multiple components of the melanocortin pathway, including PCSK1 (ref. 23 ), MC4R 24 , 25 , 26 and POMC 27 , 28 , 29 , all of which were found to result in severe early-onset obesity (Table 1 ).
Advances in high-throughput DNA sequencing led to candidate gene screening being replaced by WES, an unbiased approach that allows all coding sequences to be screened for mutations. However, it rapidly became clear that, whereas candidate gene studies yielded few mutations, WES identified too many potential obesity-associated variants such that the noise often masked the true causative mutations. However, with improved algorithms to predict the pathogenicity of mutations, as well as a rapidly expanding toolkit of functional assays, it has become easier to filter the likely pathogenic mutations. Several success stories have been reported in which WES has identified novel pathways and genes linked to obesity, such as the class 3 semaphorins (SEMA3A–G), which have been shown to direct the development of certain hypothalamic neurons, including those expressing pro-opiomelanocortin (POMC) 30 (see ‘Other neuronal circuits and molecules linked to severe obesity’).
Most monogenic obesity mutations have been identified in cohorts of patients with severe and early-onset (<10 years old) obesity. Additionally, as monogenic obesity often demonstrates a recessive inheritance pattern 31 , consanguinity in populations has further increased the chance of identifying mutations, owing to greater chances of homozygosity of deleterious mutations 32 . For example, studies have reported that mutations in the genes encoding leptin, LEPR and MC4R explain 30% of cases of severe obesity in children from a consanguineous Pakistani population 33 , and single-gene defects more broadly account for nearly 50% 34 .
Gene discovery for polygenic obesity
The discovery of genes that influence polygenic obesity, which is common in the general population, started off slowly with candidate gene studies and genome-wide linkage studies . The candidate gene approach was first applied in the mid-1990s and aimed to validate genes identified through human and animal models of extreme obesity for a role in common obesity (Fig. 3 ). Common variants in such candidate genes were tested for association with obesity risk, BMI or other body composition traits. Over the subsequent 15 years, hundreds of genes were studied as candidates, but variants in only six ( ADRB3 (ref. 35 ), BDNF 36 , CNR1 (ref. 37 ), MC4R 38 , PCSK1 (ref. 39 ) and PPARG 40 ) showed reproducible association with obesity outcomes. The genome-wide linkage approach made its entrance into the field towards the end of the 1990s (Fig. 3 ). Genome-wide linkage studies rely on the relatedness of individuals and test whether certain chromosomal regions co-segregate with a disease or trait across generations. Even though more than 80 genome-wide linkage studies identified >300 chromosomal loci with suggestive evidence of linkage with obesity traits, few loci were replicated and none was successfully fine-mapped to pinpoint the causal gene or genes 41 . Ultimately, candidate gene and genome-wide linkage studies, constrained by small sample sizes, sparse coverage of genetic variation across the genome and lack of replication, only had a marginal impact on the progression of gene discovery for common obesity outcomes.
However, the pace of gene discovery for common diseases accelerated with the advent of genome-wide association studies (GWAS) (Fig. 3 ). The first GWAS for obesity traits were published in 2007 and identified a cluster of common variants in the first intron of the FTO locus that was convincingly associated with BMI 42 , 43 . Many more GWAS followed and, to date, nearly 60 GWAS have identified more than 1,100 independent loci associated with a range of obesity traits 44 (Supplementary Tables 1 , 2 ).
As sample sizes increase with each consecutive GWAS, the statistical power to identify more loci also increases, in particular for loci that are less common and/or have smaller effects. For example, the first GWAS were relatively small ( n = ~5,000) and identified only the FTO locus 42 , 43 . The BMI-increasing allele of FTO is common, particularly in populations of European ancestry (minor allele frequency (MAF) 40–45%), and has a relatively large effect on BMI (0.35 kg m −2 per allele; equivalent to 1 kg for a person who is 1.7 m tall). Ten years and numerous GWAS later, the most recent GWAS for BMI included nearly 800,000 individuals, identified more than 750 loci, with MAFs as small as 1.6% and per-allele effects as low as 0.04 kg m −2 per allele (equivalent to 120 g for a person who is 1.7 m tall) 45 . Combined, these genome-wide significant loci explained 6% of variation in BMI 45 . Large-scale international collaborations have been formed, such as the Genetic Investigation for Anthropometric Traits (GIANT) consortium , that combine summary statistics of individual GWAS to generate data sets comprising hundreds of thousands of individuals. Furthermore, many GWAS efforts have maximized sample size by focusing on BMI as the primary obesity outcome, an inexpensive and easy-to-obtain measurement that is readily available in most studies. As such, the vast majority of loci have been identified first in GWAS of BMI, but their effects typically transfer to other overall adiposity outcomes.
Even though BMI is widely used, it is considered a crude proxy of overall adiposity because it does not distinguish between lean and fat mass 46 . Therefore, GWAS have been performed for more refined obesity traits, such as body fat percentage 47 , 48 , fat-free mass 49 , imaging-derived adipose tissue 50 , circulating leptin levels 51 and LEPR levels 52 . In addition, two GWAS have focused on persistent healthy thinness, assuming that genes that determine resistance to weight gain may also inform obesity prevention and weight loss maintenance 53 , 54 . Although GWAS of more refined and alternative obesity outcomes are generally much smaller than those for BMI, the phenotypes are often a more accurate representation of body-weight regulation and, as such, the loci identified tend to more often point to relevant biological pathways that underlie obesity.
Almost all GWAS loci for obesity outcomes were first identified in adults. Most of these loci also associate with obesity and/or BMI in children and adolescents, highlighting the fact that the genetic underpinning of obesity is relatively constant across the course of life 55 , 56 , 57 . Similarly to gene discovery for other common diseases, the obesity genetics field has suffered from a strong bias in population representation, with the vast majority of GWAS being performed in populations that are exclusively or predominantly of European ancestry. Nevertheless, some loci have first been discovered in populations of Asian 58 , African 59 , 60 , Hispanic or other ancestry 61 , despite their much smaller sample sizes. Broadly, loci identified in one ancestry demonstrate good transferability (that is, directionally consistent associations) across other ancestries, even though effect sizes and allele frequencies may differ. The modest-to-high genetic correlations across ancestries observed for BMI ( r = 0.78) are consistent with good transferability 62 , but also suggest that ancestry-specific loci remain to be discovered. Besides increasing the sample sizes of GWAS in populations of non-European ancestry, demographic, evolutionary and/or genomic features of specific populations (such as founder, consanguineous or isolated populations) have been leveraged for gene discovery, identifying genetic variants with large effects that are common in the discovery population, such as CREBRF , first identified in Samoan populations, and ADCY3 , first identified in the Greenlandic population, but rare or nonexistent in most others 63 , 64 , 65 , 66 . CREBRF has been shown to play a role in cellular energy storage and use, and may be implicated in cellular and organismal adaptation to nutritional stress 65 . ADCY3 colocalizes with MC4R at the primary cilia of a subset of hypothalamic neurons that have been implicated in body-weight regulation 67 .
GWAS have typically focused on biallelic, common genetic variation (MAF >5%), but have also been used to screen for the role of copy number variants (CNVs) in obesity. So far, only a few CNVs have been identified that have a convincing association with BMI, such as the 1p31.1 45-kb deletion near NEGR1 (ref. 68 ), which encodes a cell-adhesion molecule expressed in the brain 69 ; the 16p12.3 21-kb deletion upstream of GPRC5B 70 , which may modulate insulin secretion 71 ; the 10q11.22 CNV in PPYR1 (also known as NPY4R ) 72 , which encodes a potent anti-obesity agent known to inhibit food intake 73 ; and the 1p21.1 multi-allele CNV encompassing AMY1A 74 , which produces salivary α-amylase, a key enzyme in starch digestion 75 .
To determine the role of other types of variation in obesity, alternative genome-wide screens have been performed. For example, the impact of low-frequency and rare protein-coding variants has been tested using exome sequencing and exome array data 76 , 77 , 78 , 79 . It was speculated that low-frequency (MAF 1–5%) and rare (MAF <1%) variants would have larger effects than common variants, and thus be easier to detect. Nevertheless, even large-scale studies identified only a few robust associations for rare coding variants. For example, exome-wide screening based on array data from more than 400,000 individuals identified p.Tyr35Ter (rs13447324) in MC4R ; p.Arg190Gln (rs139215588) and p.Glu288Gly (rs143430880) in GIPR , which stimulates insulin secretion and mediates fat deposition 80 ; p.Arg95Ter (rs114285050) in GRP151 , which modulates habenular function that controls addiction vulnerability 81 ; and p.Arg769Ter (rs533623778) in PKHD1L1 , which has been involved in cancer development 77 , 78 . A recent study that leveraged WES data for more than 600,000 individuals identified 16 genes for which the burden of rare nonsynonymous variants was associated with BMI, including five brain-expressed G protein-coupled receptors ( CALCR , MC4R , GIPR , GPR151 and GPR75 ) 79 .
As obesity is a complex, multifactorial condition, some GWAS have integrated demographic factors (such as sex and age 82 ) and environmental factors (such as physical activity 83 , diet 84 or smoking 85 ) into their analyses. Despite sample sizes of more than 200,000 individuals, these genome-wide gene-by-environment (G×E) interaction analyses remain challenging and so far only 12 loci have been identified, the effects of which on obesity are attenuated or exacerbated by non-genetic factors. Nevertheless, the G×E interaction between the FTO locus and a healthy lifestyle has been robustly replicated. Specifically, increased physical activity or a healthy diet can attenuate the effect of the FTO locus on obesity risk by 30–40% 86 , 87 .
The increasing availability of large-scale cohorts and biobanks, such as the UK Biobank , the Million Veterans Project , All of Us , Biobank Japan and 23andMe , combined with ongoing work by the GIANT consortium, will boost sample sizes further to easily exceed 4 million participants in meta-analyses, expediting the discovery of many more obesity-associated loci. However, translation of GWAS-identified loci into new biological insights remains a major challenge.
From genes to biology
Despite the difficulties in validating causative mutations and variants, genetic studies into both rare and common obesity over the past two decades have revealed two surprisingly cogent, overarching biological messages: first, the leptin–melanocortin pathway is a key appetitive control circuit 31 , 88 (Fig. 4 ); and second, genes that are either enriched or exclusively expressed within the brain and CNS have a central role in obesity 89 .
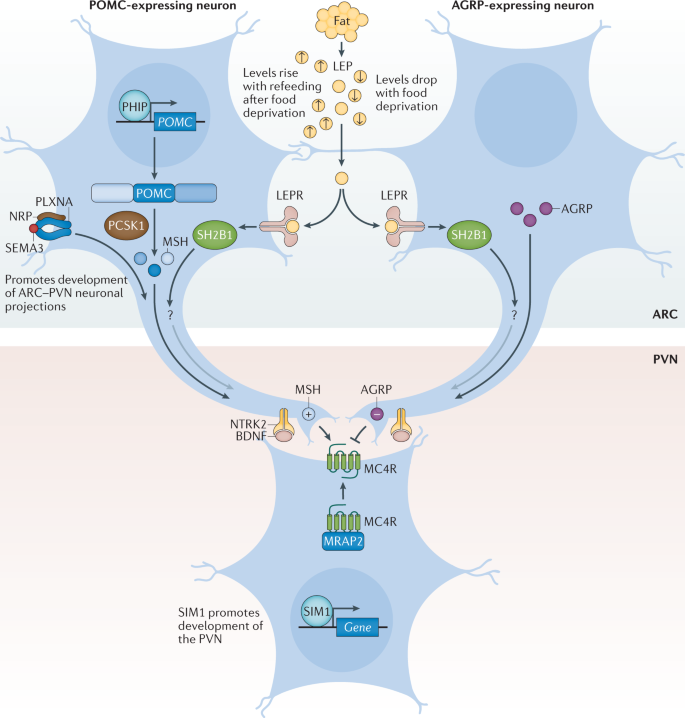
Pro-opiomelanocortin (POMC)-expressing neurons and agouti-related protein (AGRP)-expressing neurons within the arcuate nucleus of the hypothalamus (ARC) act to sense circulating leptin (LEP) levels, which reflect fat mass. These neurons signal to melanocortin 4 receptor (MC4R)-expressing neurons in the paraventricular nucleus of the hypothalamus (PVN), which controls appetite, thus linking long-term energy stores to feeding behaviour. Binding of class 3 semaphorins (SEMA3) to their receptors NRP and PLXNA influences the projection of POMC neurons to the PVN. Binding of brain-derived neurotrophic factor (BDNF) to its receptor neurotrophic receptor tyrosine kinase 2 (NTRK2) is thought to be an effector of leptin-mediated synaptic plasticity of neurons, including those in the ARC and PVN. The transcription factor SIM1 is crucial for the proper development of the PVN. +, agonist; −, antagonist; LEPR, leptin receptor; MRAP2, melanocortin receptor accessory protein 2; MSH, melanocyte-stimulating hormone; SH2B1, SH2B adaptor protein 1.
The leptin–melanocortin pathway and MC4R
Leptin is a key hormone secreted by adipocytes, which circulates at levels in proportion to fat mass 90 . Leptin also responds to acute changes in energy state, as its levels decrease with food deprivation and are restored during re-feeding. Administration of leptin to fasted mice abrogates many of the neuroendocrine consequences of starvation, suggesting that the normal biological role of leptin is to initiate the starvation response 91 . Leptin signals through the LEPR, which exists in several different isoforms. However, obesity-related effects of leptin are predominantly mediated by a long isoform that contains an intracellular domain (LEPRb), which is expressed in various regions of the CNS 90 .
Within the arcuate nucleus (ARC) of the hypothalamus, LEPRb is found on two populations of neurons at the heart of the melanocortin pathway, one of which expresses POMC and the other agouti-related protein (AGRP) 92 (Fig. 4 ). POMC is post-translationally processed by prohormone convertases to produce several biologically active moieties, including β-lipotrophin and β-endorphin, and, crucially, the melanocortin peptides adrenocorticotrophin (ACTH) and α-, β- and γ-melanocyte-stimulating hormone (MSH) 93 . The ARC POMC neurons project to MC4R neurons within the paraventricular nucleus (PVN) where melanocortin peptides signal to decrease food intake 92 . By contrast, AGRP acts as an endogenous antagonist of MC4R to increase food intake 92 , 94 . MC3R is another centrally expressed receptor that binds to both melanocortin peptides and AGRP; however, as mice with targeted deletions in the gene are not obese but instead have altered fat to lean mass ratio, MC3R is less likely to be related to food intake and more likely to be involved in nutrient partitioning 95 , 96 .
We can state with confidence that the fine balance of melanocortinergic agonism and AGRP antagonism of MC4R, in response to peripheral nutritional cues such as leptin, plays a central part in influencing appetitive drive 92 . The genetic evidence clearly supports this contention, with mutations in most genes of the melanocortin pathway resulting in hyperphagia and severe obesity in both humans and mice 31 , 88 . In fact, the vast majority of single-gene disruptions causing severe early-onset obesity in humans fall within this pathway, including LEPR , POMC , AGRP , MCR4R , PCSK1 (ref. 23 ), SH2B1 (ref. 97 ), PHIP 98 , MRAP2 (ref. 99 ) and SIM1 (ref. 100 ) (Fig. 4 ; Table 1 ). Mutations in MC4R in particular, are the most common single-gene defect leading to hyperphagia and obesity. Pathogenic mutations in MC4R are found in up to 5% of cases of severe childhood obesity 101 and up to 0.3% of the general population 101 , 102 . Of note, the degree of receptor dysfunction, as measured by in vitro assays, can predict the amount of food eaten at a test meal by an individual harbouring that particular mutation 101 . Thus MC4R does not act in a binary on/off manner, but as a rheostat; put simply, the melanocortin pathway is a ‘tunable’ system. In addition to regulating food intake, it also regulates food preference, with individuals who carry mutations in MC4R showing a preference for food with higher fat content 103 .
The importance of the melanocortin pathway in regulating feeding behaviour is highlighted by the identification of naturally occurring mutations in pathway genes in a wide range of different species where the appropriate selection pressure has been present (Table 1 ). For example, studies have found that 20–25% of Labrador retrievers, which are known to be more food-motivated than other dog breeds, carry a 14-bp deletion in POMC that disrupts the β-MSH and β-endorphin coding sequences and is associated with greater food motivation and increased body weight 104 . Also, certain breeds of pig have been shown to carry MC4R missense mutations that are associated with fatness, growth and food intake traits 105 . MC4R mutations even contribute to the adaptation and survival of blind Mexican cavefish to the nutrient-poor conditions of their ecosystem 106 .
Other neuronal circuits and molecules linked to severe obesity
It is now clear that in addition to engaging classical neuropeptide–receptor systems within the brain, leptin also rapidly modifies synaptic connections between neurons 107 , and that this structural plasticity is crucial to its downstream functions. One of the ways in which this plasticity is thought to be achieved is via brain-derived neurotrophic factor (BDNF) signalling to its receptor TrkB. BDNF is widely expressed in the CNS where it plays an important part in neuronal development 108 , 109 . In the hippocampus, BDNF contributes to synaptic plasticity and long-term potentiation associated with memory and learning 110 . However, evidence has emerged that implicates BDNF and TrkB in the regulation of mammalian eating behaviour and energy balance 111 . BDNF is downregulated by nutritional deprivation and upregulated by leptin within the ventromedial nucleus (VMN) of the hypothalamus 112 , although this regulation is probably indirect, as very few VMN BDNF neurons express the LEPR 113 (Fig. 4 ) and some evidence indicates that it acts at least in part downstream of melanocortin signalling 112 . Crucially, genetic disruption of BDNF 114 , 115 and TrkB 112 , 116 in both humans and mice results in hyperphagia and severe obesity.
Another group of neuronal proteins important in the development of neuronal circuitry and linked to energy balance are the class 3 semaphorins (SEMA3A–G). A study in humans found that 40 rare loss-of-function variants in SEMA3A–G and their receptors (PLXNA1–4, NRP1 and NRP2) were significantly enriched in 982 individuals with severe obesity compared with 4,449 controls 30 . Disruption of several of these genes in zebrafish caused increased somatic growth and/or adiposity, and experiments with mouse hypothalamic explants suggest that SEMA3 signalling via NRP2 receptors drives the development of POMC projections from the ARC to the PVN 30 . However, given that these results are from a single study, more data are required to confirm the exact role of class 3 semaphorins in energy homeostasis.
Insights from genetic loci linked to common obesity
Unlike candidate gene studies, GWAS make no a priori assumptions about the underlying biology that links genetic variants to a disease of interest. While this agnostic approach allows for new biological insights, the vast majority of GWAS-identified variants map to the non-coding parts of genes or to regions between genes. As such, they do not directly disrupt the protein-coding regions, but instead overlap with regulatory elements that influence expression of genes in close proximity or even over long distances.
However, even if the causative genes are unknown, pathway, tissue and functional enrichment analyses based on the genes located in the GWAS loci can provide insights into potential mechanisms. Since the very first GWAS for BMI 68 , 117 , such analyses have pointed to the CNS being a key player in body-weight regulation, consistent with insights from human and animal models of extreme obesity. Recent analyses that include the latest BMI-associated loci, combined with updated multi-omics databases and advanced computational tools, have further refined these observations. In addition to the hypothalamus and pituitary gland (which are both known appetite regulation sites), other brain areas have been highlighted, including the hippocampus and the limbic system (which are involved in learning, cognition and emotion) and the insula and the substantia nigra (which are related to addiction and reward) 58 , 89 , 118 , 119 . The enrichment of immune-related cells (such as lymphocytes and B cells) and adipose tissue was found to be weaker 58 .
Although enrichment analyses provide preliminary insights into the broad biology represented by genes in the GWAS loci, determining which genes, variants and/or underlying mechanisms are causal has proved an arduous task. For example, the FTO locus, which was identified more than a decade ago and harbours six genes, is the most extensively studied GWAS-identified obesity locus (Fig. 5 ). Despite its highly significant and widely replicated association with obesity 120 , the causal variants and/or genes in the FTO locus have not yet been pinpointed with convincing evidence, and the mechanisms by which the locus affects body weight have not been fully elucidated. Early functional follow-up analyses suggested that FTO itself might be responsible, as Fto deficiency in mice results in a lean phenotype, whereas Fto overexpression is associated with increased body weight 121 , 122 . Studies in mice have suggested that FTO plays a role in cellular nutrient sensing 123 , 124 . Other studies found evidence that FTO influences brain regions that affect appetite, reward processing and incentive motivation by regulating ghrelin levels in humans 125 or by controlling dopaminergic signalling in mice 126 , 127 . In addition, variants in the FTO locus were shown to alter a regulatory element that controls the transcription of Rpgrip1l in mice, a ciliary gene located immediately upstream of Fto 128 , 129 , 130 . Mice with reduced Rpgrip1l activity exhibit hyperphagic obesity, possibly mediated through diminished leptin signalling 128 , 129 , 130 . In recent years, studies in human and animal models have shown that variants in the FTO locus directly interact with the promoter of Irx3 , a gene located 0.5 Mb downstream of FTO . Irx3 -deficient mice were found to exhibit weight loss and increased metabolic rate with browning of white adipose tissue, without changes in physical activity or appetite 131 , 132 . Further in-depth functional characterization showed that rs1421085 in the FTO locus disrupts a conserved binding motif for the transcriptional repressor ARID5B, which leads to a doubling of IRX3 and IRX5 expression during early adipocyte differentiation 132 . The authors argue that increased expression of these genes results in a developmental shift from energy-dissipating beige adipocytes to energy-storing white adipocytes, a fivefold reduction in mitochondrial thermogenesis and increased lipid storage 132 . However, given that multiple studies have shown that the FTO locus is robustly associated with food intake, with no evidence to date linking it to changes in energy expenditure, the relevance of this observation to the actual observed human phenotype still needs to be explored 133 . A recent study reports that the FTO locus affects gene expression in multiple tissues, including adipose tissue and brain, and, more broadly, that the genetic architecture of disease-associated loci may involve extensive pleiotropy and allelic heterogeneity across tissues 134 .
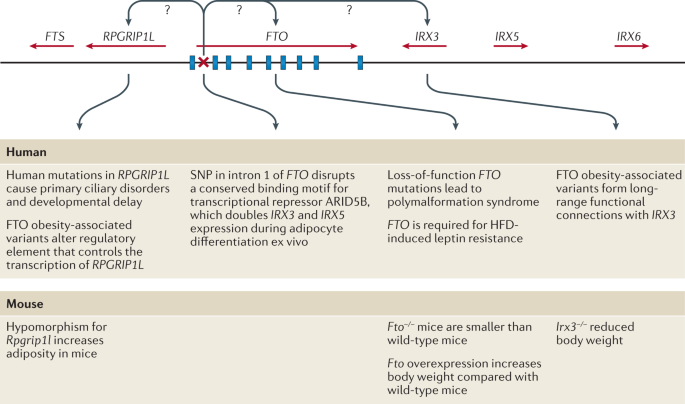
FTO contains nine exons (depicted by blue rectangles) and the body mass index (BMI)-associated SNP identified in genome-wide association studies (depicted by a red ×) maps to intron 1. IRX3 and RPGRIP1L have both been proposed to be the causal genes for obesity within the locus and to act on body weight through distinct mechanisms. HFD, high-fat diet.
Besides the FTO locus, functional follow-up analyses have been performed for only a few obesity-associated GWAS loci. For example, early studies identified a cluster of variants just downstream of TMEM18 (refs 68 , 117 ). TMEM18 encodes a poorly characterized transmembrane protein that is highly conserved across species and widely expressed across tissues, including in several regions of the brain 135 , 136 . Tmem18 deficiency in mice results in a higher body weight owing to increased food intake, whereas Tmem18 overexpression reduces food intake and limits weight gain 136 . A knockdown experiment in Drosophila melanogaster suggests that TMEM18 affects carbohydrate and lipid levels by disrupting insulin and glucagon signalling 137 .
Two other GWAS loci for which functional analyses have been performed are located just upstream of CADM1 (ref. 82 ) and in CADM2 (ref. 70 ), genes that encode cell-adhesion proteins of the immunoglobulin superfamily and mediate synaptic assembly in the CNS 138 . The BMI-increasing alleles at each locus are associated with increased expression of CADM1 and CADM2 in the hypothalamus 139 , 140 . Deficiency of either Cadm1 or Cadm2 in mice results in a lower body weight and increased insulin sensitivity, glucose tolerance and energy expenditure without any change in food intake 139 , 140 . Conversely, increased neuronal expression of either Cadm1 or Cadm2 is associated with elevated body weight 139 , 140 . Furthermore, CADM1 is expressed in POMC neurons and Cadm1 deficiency leads to an increase in the number of excitatory synapses, suggestive of an increased synaptic plasticity 140 . Cadm2 -deficient mice exhibit increased locomotor activity and higher core body temperature 139 .
Another GWAS locus, just upstream of NEGR1 , harbours two deletions associated with increased obesity risk 68 , 117 , 141 . These deletions do not overlap with the coding sequence of NEGR1 , but encompass a conserved transcription factor-binding site for NKX6.1 , a potent transcriptional repressor 68 , 141 . Loss of binding of NKX6.1 leads to higher NEGR1 expression 141 , which is consistent with the observation that BMI-increasing alleles (that is, deletions) at this locus are associated with higher NEGR1 expression in the brain. Similar to CADM1 and CADM2, NEGR1 is a cell-adhesion molecule of the immunoglobulin superfamily that is expressed in several regions of the brain and has been shown to have a role in brain connectivity 69 , 142 , a process believed to be important in obesity 143 . NEGR1 deficiency in mice was shown to result in lower body weight, mainly due to reduced lean mass, mediated by lower food intake 144 . However, two other functional studies, one in mice and one in rats, found that knockdown of Negr1 expression resulted in the opposite phenotype — increased body weight and food intake 145 , 146 . While NEGR1 deficiency in mice was found to impair core behaviours, so far, findings and proposed mechanisms are not fully aligned 69 , 147 , 148 , 149 .
Taken together, functional follow-up analyses for these loci are slowly expanding our understanding of the pathophysiology that drives weight gain. However, many more obesity-associated loci are waiting to be translated into new biological insights. A major hurdle in translating GWAS loci into plausible candidate genes and appropriate paradigms for functional research is the annotation of the associated variants in a locus. Defining the regulatory function of the non-coding variants, identifying their putative effector transcripts and determining their tissues of action remains an ongoing challenge. The advent of high-throughput genome-scale technologies for mapping regulatory elements, combined with comprehensive multi-omics databases, advanced computational tools and the latest genetic engineering and molecular phenotyping approaches, is poised to speed up the translation of GWAS loci into meaningful biology 150 .
Converging results from monogenic and polygenic forms of obesity
Gene discovery is often dichotomized by allele frequency and disease prevalence; that is, mutations are sought for monogenic forms of obesity and common variants for polygenic obesity (Fig. 2 ). However, it is increasingly recognized that monogenic and polygenic forms of obesity are not discrete entities. Instead, they lie on a spectrum and share — at least in part — the same biology. As GWAS have continued to discover more obesity-associated loci, an increasing number of these loci harbour genes that were first identified for extreme and early-onset obesity in humans or animal models, including MC4R 151 , 152 , BDNF 117 , SH2B1 (refs 68 , 117 ), POMC 70 , LEP 51 , 153 , LEPR 52 , 154 , NPY 155 , SIM1 (ref. 155 ), NTRK2 (ref. 58 ), PCSK1 (ref. 154 ) and KSR2 (ref. 77 ). In fact, most of these genes encode components of the leptin–melanocortin and BDNF–TrkB signalling pathways (Table 1 ). Thus, whereas genetic disruption of components of these pathways results in severe obesity, genetic variants in or near these same genes that have more subtle effects on their expression will influence where an individual might sit in the normal distribution of BMI.
Although most genes have been first identified for extreme forms of obesity, a locus harbouring ADCY3 was first identified in GWAS for common obesity 77 , and ADCY3 was subsequently confirmed as having a role in extreme obesity 63 , 64 . ADCY3 encodes an adenylate cyclase that catalyses the synthesis of cAMP, an important second messenger in signalling pathways. There is some evidence that ADCY3 (adenylate cyclase) colocalizes with MC4R at the primary cilia of PVN neurons 67 and that cilia are required specifically on MC4R-expressing neurons for the control of energy homeostasis 156 . In mice, disruption of Adcy3 or Mc4r in the cilia of these neurons impairs melanocortin signalling, resulting in hyperphagia and obesity 67 .
As more GWAS loci are reported, we expect that findings across different lines of obesity research will continue to converge, providing accumulating evidence for new biology.
From genes to clinical care
Genetic insights from gene discovery efforts are increasingly being used in the context of precision medicine in ways that directly affect health. Knowing a patient’s genotype may enable a more precise diagnosis of the type of obesity, which in turn allows the prescription of personalized treatment or prevention strategies. Furthermore, knowing an individual’s genetic susceptibility to obesity early in life may help to more accurately predict those most at risk of gaining weight in the future.
Use of genotype information in treatment of obesity
When a disease is caused by a single mutation and the environmental contribution is limited, as is the case for some forms of extreme and early-onset obesity, a genetic test can be instrumental in correctly diagnosing patients. Although no standard genetic testing panel is currently available for extreme and early-onset obesity, some clinics, research centres and pharmaceutical companies sequence well-known candidate genes to identify the functional mutation that may be the cause of a patient’s excess body weight. Such a genetic diagnosis can lessen the feelings of guilt and blame for the patient, and alleviate social stigma and discrimination. Importantly, a genetic diagnosis can inform disease prognosis and, in some cases, it will determine treatment. To date, there are two treatments for obesity that are tailored to patient genotype.
The prototype of genotype-informed treatment for obesity is the administration of recombinant human leptin in patients who are leptin-deficient owing to mutations in the LEP gene 157 , 158 . Although congenital leptin deficiency is exceptionally rare (only 63 cases have been reported to date 28 ), leptin replacement therapy has been remarkably beneficial for these patients by substantially reducing food intake, body weight and fat mass, and normalizing endocrine function 157 , 158 . It has literally transformed their lives.
The second genotype-informed treatment for obesity is setmelanotide, a selective MC4R agonist that was recently approved by the FDA for rare monogenic obesity conditions including LEPR, PCSK1 and POMC deficiency 159 . Setmelanotide acts as a substitute for the absent MSH in patients with POMC deficiency owing to mutations in POMC or PCSK1 , and in patients with LEPR deficiency owing to mutations in LEPR , which is essential for POMC function 160 , 161 , 162 . Daily subcutaneous injection of setmelanotide results in substantial weight loss and in reduction of hunger 160 , 161 , 162 . After a 1-year treatment with setmelanotide in phase III trials, patients with POMC deficiency lost on average 25.6% of their initial weight, with 80% of patients achieving at least a 10% weight loss 162 . The adverse effects of setmelanotide treatment are minor, and include hyperpigmentation, nausea and/or vomiting, penile erection and injection site reactions. Weight loss in patients with LEPR deficiency was less pronounced; on average, they lost 12.5% of their initial weight, with only 45% of patients achieving at least a 10% weight loss 162 . The difference in weight loss between the two patient groups may be because POMC deficiency directly affects the production of MC4R ligands (α-MSH and β-MSH), whereas LEPR deficiency affects signalling upstream of POMC 162 . As such, setmelanotide may be able to completely restore MC4R signalling in POMC deficiency, but only partially in LEPR deficiency. Even though the average weight loss in POMC-deficient patients was twice that in LEPR-deficient patients, the reduction in hunger was substantially larger in LEPR-deficient patients (−43.7%) than in POMC-deficient patients (−27.1%) 162 . The reasons for the discrepancy between weight loss and reduction in hunger remain to be studied in greater depth. It has been estimated that in the USA, >12,800 individuals carry mutations in the melanocortin pathway for whom setmelanotide may be more effective for weight loss than any other treatment 163 . Although 12,800 carriers represent only a fraction (0.004%) of the adult population in the USA, and not all of these mutation carriers are overweight or obese, for the patients for whom setmelanotide is effective, it may end a lifelong battle to lose weight 163 . In patients without genetic defects, neither setmelanotide nor leptin administration have, to date, demonstrated a substantial effect on weight loss 164 , 165 .
These two genotype-informed treatments show how insight into the underlying biological mechanisms can guide the development of molecules and medications that restore impaired pathways, at least in monogenic forms of obesity caused by deficiency of one protein. Nevertheless, there remain substantial obstacles in the transition from conventional to precision medicine for monogenic obesity, which would require the adoption of systematic WES for individuals suspected to be carriers of deleterious mutations, and eventually even standardized screening at birth. We are clearly a long way from such a scenario at present.
Use of genotype information in prediction of obesity
As more variants are being discovered for common obesity, there is a growing expectation that genetic information will soon be used to identify individuals at risk of obesity. Knowing a person’s genetic susceptibility would allow for a more accurate prediction of who is at risk of gaining weight and give an opportunity to intervene earlier to prevent obesity more effectively. Genetic susceptibility to complex disease, including obesity, is assessed using a polygenic score (PGS). PGSs to assess obesity susceptibility are based on GWAS for BMI (PGS BMI ), the latest of which includes data on more than 2 million variants and explains 8.4% of the variation in BMI 166 . The average BMI of individuals with a high PGS BMI (top decile) is 2.9 kg m −2 (equivalent to 8 kg in body weight) higher and their odds of severe obesity (BMI ≥40 kg m −2 ) is 4.2-fold higher than those with a lower PGS BMI (lowest nine deciles) 166 .
Despite these strong associations with BMI and obesity, the predictive performance of the PGS BMI is weak, which is unsurprising given its limited explained variance. For example, using the same PGS BMI and data from the UK Biobank, we estimate that the area under the receiver operating characteristic curve (AUC ROC ) is only 0.64 to predict obesity. This means that the probability that an individual with obesity has a higher PGS BMI than an individual without obesity is 0.64. However, for a PGS to have clinical utility, the AUC ROC needs to be much higher (>0.80). In addition, we calculated the extent to which a PGS BMI ≥90th percentile correctly classifies individuals with obesity (Fig. 6 ). We found that such a predictive test (PGS BMI ≥90th percentile) has a positive predictive value of 0.43, meaning that of those who were predicted to develop obesity, only 43% actually developed obesity. Its sensitivity is 0.19, which means that of the individuals who developed obesity, only 19% had been correctly classified by the PGS BMI . Given that the current treatment options for obesity are low risk, or even generally beneficial, the high false-positive rate is less concerning than the low sensitivity, as some at-risk individuals may miss the opportunity for early prevention.
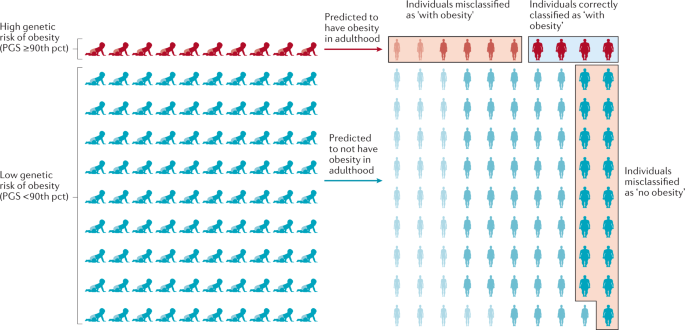
The outcome is illustrated for a polygenic score (PGS) that assumes that individuals with a score in the highest decile (≥90th percentile (pct)) will develop obesity, has a positive predictive value of 0.4 and a sensitivity of 0.19. Of ten individuals with a high score classified by the PGS as ‘with obesity’, four will be classified correctly but the other six will be misclassified and will not develop obesity — a positive predictive value of 0.4. Likewise, 17 of the 90 individuals with a score <90th pct who are predicted to not develop obesity, will develop obesity. Thus, only four of the 21 individuals who developed obesity were correctly classified by the PGS — a sensitivity of 0.19. Misclassified individuals are indicated by the red boxes, individuals correctly classified as ‘with obesity’ are indicated by a blue box. Adapted with permission from ref. 170 , Elsevier.
Thus, the current PGS BMI has a high rate of misclassification and does not reliably predict who is at risk of developing obesity and who is not. The predictive ability of PGSs are expected to improve as GWAS increase in sample size and algorithms to calculate the scores become more refined. Nevertheless, given the importance of socio-demographic, lifestyle and clinical risk factors in the aetiology of obesity, it is unlikely that a PGS BMI will ever be able to accurately predict obesity on its own. Instead, effective prediction models will have to include genetic and non-genetic factors, including a broad spectrum of demographic, environmental, clinical and possibly molecular markers, as well.
Conclusions and future perspectives
What initially began as two apparently distinct approaches, one studying rare Mendelian causes of extreme obesity, and the other exploring complex polygenic influences of population body-weight distribution, have eventually converged on the central role of the brain in regulating body weight. In particular, both approaches have highlighted the roles of the leptin–melanocortin pathway and TrkB–BDNF signalling. Perhaps it seems obvious now, but it was by no means certain that, just because genetic disruption of a pathway resulted in a severe phenotype, polymorphisms within that same pathway would produce a more subtle and nuanced result.
The GWAS approach is hypothesis-free, with the promise to reveal new genes that point to new biology and pathways. However, for the vast majority of the >1,000 GWAS-identified loci, we do not know which genes are causal, what cells, tissues and organs they act in to affect body weight, and we do not understand the underlying mechanisms. The translation from variant to function is a well-known challenge 167 , but with increasing availability of new omics data, high-throughput technologies and advanced analytical approaches, there is an unprecedented opportunity to speed up the translation of hundreds of GWAS loci.
Sample size remains a major driver for gene discovery. In an ongoing collaboration that combines data from more than 3 million individuals of diverse ancestry from the GIANT consortium, the UK Biobank and 23andMe, the number of BMI-associated GWAS loci is set to double. Also, a recent WES effort of more than 640,000 individuals has demonstrated that rare mutations are discoverable when sample sizes are sufficiently large 79 . However, alternative study designs, a focus on more refined phenotypes or a focus on population subgroups (that is, more homogeneous groups of individuals with similar outcomes) could further add to gene discovery.
Translation of only a few dozen of the GWAS-identified loci could tremendously improve our insights into the biology of obesity and possibly reveal new therapeutic targets. It would also take us a little closer to the ‘holy grail’ — the ability to move away from a failed ‘one-size-fits-all’ strategy, and towards true precision medicine for obesity, metabolic disease and other diet-related illnesses.
GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 377 , 13–27 (2017).
Article Google Scholar
Must, A. et al. The disease burden associated with overweight and obesity. JAMA 282 , 1523–1529 (1999).
Article CAS PubMed Google Scholar
Fontaine, K. R. & Barofsky, I. Obesity and health-related quality of life. Obes. Rev. 2 , 173–182 (2001).
Bhattacharya, I., Ghayor, C., Perez Dominguez, A. & Weber, F. E. From influenza virus to novel corona virus (SARS-CoV-2)—the contribution of obesity. Front. Endocrinol. 11 , 556962 (2020).
Petrilli, C. M. et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ 369 , m1966 (2020).
Article PubMed PubMed Central Google Scholar
Cummings, M. J. et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet 395 , 1763–1770 (2020).
Article CAS PubMed PubMed Central Google Scholar
Zhao, X. et al. Obesity increases the severity and mortality of influenza and COVID-19: a systematic review and meta-analysis. Front. Endocrinol. 11 , 595109 (2020).
Abarca-Gómez, L. et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 390 , 2627–2642 (2017).
Maes, H. H., Neale, M. C. & Eaves, L. J. Genetic and environmental factors in relative body weight and human obesity. Behav. Genet. 27 , 325–351 (1997).
Elks, C. E. et al. Variability in the heritability of body mass index: a systematic review and meta-regression. Front. Endocrinol. 3 , 29 (2012). This paper reports a large-scale meta-analysis of heritability data of twin and family studies .
Chami, N., Preuss, M., Walker, R. W., Moscati, A. & Loos, R. J. F. The role of polygenic susceptibility to obesity among carriers of pathogenic mutations in MC4R in the UK Biobank population. PLoS Med. 17 , e1003196 (2020). This study shows that the effect of MC4R mutations on BMI and obesity risk is attenuated by the polygenic background .
Kaur, Y., de Souza, R. J., Gibson, W. T. & Meyre, D. A systematic review of genetic syndromes with obesity. Obes. Rev. 18 , 603–634 (2017).
Ingalls, A. M., Dickie, M. M. & Snell, G. D. Obese, a new mutation in the house mouse. J. Hered. 41 , 317–318 (1950).
Hummel, K. P., Dickie, M. M. & Coleman, D. L. Diabetes, a new mutation in the mouse. Science 153 , 1127–1128 (1966).
Zhang, Y. et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372 , 425–432 (1994). This paper reports the cloning of leptin, which provided the first molecular evidence that feeding and regulation of body weight had a hormonal basis .
Article CAS Google Scholar
Chen, H. et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db / db mice. Cell 84 , 491–495 (1996). This paper reports the cloning of the LEPR and the localization of its signalling version, which highlights the role of the brain in the control of appetitive behaviour .
Bultman, S. J., Michaud, E. J. & Woychik, R. P. Molecular characterization of the mouse agouti locus. Cell 71 , 1195–1204 (1992).
Miller, M. W. et al. Cloning of the mouse agouti gene predicts a secreted protein ubiquitously expressed in mice carrying the lethal yellow mutation. Genes Dev. 7 , 454–467 (1993).
Lu, D. et al. Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature 371 , 799–802 (1994).
Fan, W., Boston, B. A., Kesterson, R. A., Hruby, V. J. & Cone, R. D. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 385 , 165–168 (1997).
Montague, C. T. et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 387 , 903–908 (1997). This work demonstrates that leptin signalling is also relevant in the human context, and provides early evidence, together with mutations in PCSK1 , of a monogenic cause of severe human obesity .
Clement, K. et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature 392 , 398–401 (1998). This work confirms the observations from mice about leptin signalling to the brain .
Jackson, R. S. et al. Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nat. Genet. 16 , 303–306 (1997). This study, together with mutations in the leptin gene, provides early evidence of a monogenic cause of severe human obesity, and also highlights the role of the nascent melanocortin pathway in body-weight regulation .
Huszar, D. et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88 , 131–141 (1997).
Yeo, G. S. et al. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat. Genet. 20 , 111–112 (1998).
Vaisse, C., Clement, K., Guy-Grand, B. & Froguel, P. A frameshift mutation in MC4R is associated with a dominant form of obesity. Nat. Genet. 20 , 113–114 (1998). This paper, together with Yeo et al. (1998), shows that heterozygous mutations in MC4R result in severe human obesity, establishing the role of the central melanocortin pathway in regulating human appetitive behaviour .
Krude, H. et al. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat. Genet. 19 , 155–157 (1998). This paper provides direct evidence that melanocortin peptides play a key role in the regulation of energy homeostasis .
Yaswen, L., Diehl, N., Brennan, M. B. & Hochgeschwender, U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat. Med. 5 , 1066–1070 (1999).
Challis, B. G. et al. Mice lacking pro-opiomelanocortin are sensitive to high-fat feeding but respond normally to the acute anorectic effects of peptide-YY(3-36). Proc. Natl Acad. Sci. USA 101 , 4695–4700 (2004).
van der Klaauw, A. A. et al. Human semaphorin 3 variants link melanocortin circuit development and energy balance. Cell 176 , 729–742.e18 (2019).
Farooqi, S. & O’Rahilly, S. Genetics of obesity in humans. Endocr. Rev. 27 , 710–718 (2006).
Saeed, S., Arslan, M. & Froguel, P. Genetics of obesity in consanguineous populations: toward precision medicine and the discovery of novel obesity genes. Obesity 26 , 474–484 (2018).
Article PubMed Google Scholar
Saeed, S. et al. Genetic variants in LEP, LEPR, and MC4R explain 30% of severe obesity in children from a consanguineous population. Obesity 23 , 1687–1695 (2015).
Saeed, S. et al. Genetic causes of severe childhood obesity: a remarkably high prevalence in an inbred population of Pakistan. Diabetes 69 , 1424–1438 (2020).
Kurokawa, N. et al. The ADRB3 Trp64Arg variant and BMI: a meta-analysis of 44 833 individuals. Int. J. Obes. 32 , 1240–1249 (2008).
Shugart, Y. Y. et al. Two British women studies replicated the association between the Val66Met polymorphism in the brain-derived neurotrophic factor (BDNF) and BMI. Eur. J. Hum. Genet. 17 , 1050–1055 (2009).
Benzinou, M. et al. Endocannabinoid receptor 1 gene variations increase risk for obesity and modulate body mass index in European populations. Hum. Mol. Genet. 17 , 1916–1921 (2008).
Wang, D. et al. Association of the MC4R V103I polymorphism with obesity: a Chinese case–control study and meta-analysis in 55,195 individuals. Obesity 18 , 573–579 (2010).
Nead, K. T. et al. Contribution of common non-synonymous variants in PCSK1 to body mass index variation and risk of obesity: a systematic review and meta-analysis with evidence from up to 331 175 individuals. Hum. Mol. Genet. 24 , 3582–3594 (2015).
Tonjes, A., Scholz, M., Loeffler, M. & Stumvoll, M. Association of Pro12Ala polymorphism in peroxisome proliferator-activated receptor gamma with pre-diabetic phenotypes: meta-analysis of 57 studies on nondiabetic individuals. Diabetes Care 29 , 2489–2497 (2006).
Rankinen, T. et al. The human obesity gene map: the 2005 update. Obesity 14 , 529–644 (2006).
Frayling, T. M. et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316 , 889–894 (2007). This paper describes the first GWAS (for type 2 diabetes) to identify a locus ( FTO ) robustly associated with BMI .
Scuteri, A. et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 3 , e115 (2007).
Buniello, A. et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 47 , D1005–D1012 (2019).
Yengo, L. et al. Meta-analysis of genome-wide association studies for height and body mass index in approximately 700 000 individuals of European ancestry. Hum. Mol. Genet. 27 , 3641–3649 (2018).
Javed, A. et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity in children and adolescents: a systematic review and meta-analysis. Pediatr. Obes. 10 , 234–244 (2015).
Kilpelainen, T. O. et al. Genetic variation near IRS1 associates with reduced adiposity and an impaired metabolic profile. Nat. Genet. 43 , 753–760 (2011).
Lu, Y. et al. New loci for body fat percentage reveal link between adiposity and cardiometabolic disease risk. Nat. Commun. 7 , 10495 (2016).
Zillikens, M. C. et al. Large meta-analysis of genome-wide association studies identifies five loci for lean body mass. Nat. Commun. 8 , 80 (2017).
Chu, A. Y. et al. Multiethnic genome-wide meta-analysis of ectopic fat depots identifies loci associated with adipocyte development and differentiation. Nat. Genet. 49 , 125–130 (2017).
Kilpelainen, T. O. et al. Genome-wide meta-analysis uncovers novel loci influencing circulating leptin levels. Nat. Commun. 7 , 10494 (2016).
Sun, Q. et al. Genome-wide association study identifies polymorphisms in LEPR as determinants of plasma soluble leptin receptor levels. Hum. Mol. Genet. 19 , 1846–1855 (2010).
Riveros-McKay, F. et al. Genetic architecture of human thinness compared to severe obesity. PLoS Genet. 15 , e1007603 (2019).
Orthofer, M. et al. Identification of ALK in thinness. Cell 181 , 1246–1262.e22 (2020).
Bradfield, J. P. et al. A trans-ancestral meta-analysis of genome-wide association studies reveals loci associated with childhood obesity. Hum. Mol. Genet. 28 , 3327–3338 (2019).
Felix, J. F. et al. Genome-wide association analysis identifies three new susceptibility loci for childhood body mass index. Hum. Mol. Genet. 25 , 389–403 (2016).
Vogelezang, S. et al. Novel loci for childhood body mass index and shared heritability with adult cardiometabolic traits. PLoS Genet. 16 , e1008718 (2020).
Akiyama, M. et al. Genome-wide association study identifies 112 new loci for body mass index in the Japanese population. Nat. Genet. 49 , 1458–1467 (2017).
Ng, M. C. Y. et al. Discovery and fine-mapping of adiposity loci using high density imputation of genome-wide association studies in individuals of African ancestry: African Ancestry Anthropometry Genetics Consortium. PLoS Genet. 13 , e1006719 (2017).
Gurdasani, D. et al. Uganda genome resource enables insights into population history and genomic discovery in Africa. Cell 179 , 984–1002.e36 (2019).
Wojcik, G. L. et al. Genetic analyses of diverse populations improves discovery for complex traits. Nature 570 , 514–518 (2019).
Martin, A. R. et al. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat. Genet. 51 , 584–591 (2019).
Grarup, N. et al. Loss-of-function variants in ADCY3 increase risk of obesity and type 2 diabetes. Nat. Genet. 50 , 172–174 (2018).
Saeed, S. et al. Loss-of-function mutations in ADCY3 cause monogenic severe obesity. Nat. Genet. 50 , 175–179 (2018).
Minster, R. L. et al. A thrifty variant in CREBRF strongly influences body mass index in Samoans. Nat. Genet. 48 , 1049–1054 (2016).
Andersen, M. K. et al. The derived allele of a novel intergenic variant at chromosome 11 associates with lower body mass index and a favorable metabolic phenotype in Greenlanders. PLoS Genet. 16 , e1008544 (2020).
Siljee, J. E. et al. Subcellular localization of MC4R with ADCY3 at neuronal primary cilia underlies a common pathway for genetic predisposition to obesity. Nat. Genet. 50 , 180–185 (2018).
Willer, C. J. et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat. Genet. 41 , 25–34 (2009).
Singh, K. et al. Neural cell adhesion molecule Negr1 deficiency in mouse results in structural brain endophenotypes and behavioral deviations related to psychiatric disorders. Sci. Rep. 9 , 5457 (2019).
Speliotes, E. K. et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat. Genet. 42 , 937–948 (2010).
Soni, A., Amisten, S., Rorsman, P. & Salehi, A. GPRC5B a putative glutamate-receptor candidate is negative modulator of insulin secretion. Biochem. Biophys. Res. Commun. 441 , 643–648 (2013).
Jarick, I. et al. Novel common copy number variation for early onset extreme obesity on chromosome 11q11 identified by a genome-wide analysis. Hum. Mol. Genet. 20 , 840–852 (2011).
Sainsbury, A. et al. Synergistic effects of Y2 and Y4 receptors on adiposity and bone mass revealed in double knockout mice. Mol. Cell Biol. 23 , 5225–5233 (2003).
Falchi, M. et al. Low copy number of the salivary amylase gene predisposes to obesity. Nat. Genet. 46 , 492–497 (2014).
Meisler, M. H. & Ting, C. N. The remarkable evolutionary history of the human amylase genes. Crit. Rev. Oral Biol. Med. 4 , 503–509 (1993).
Hendricks, A. E. et al. Rare variant analysis of human and rodent obesity genes in individuals with severe childhood obesity. Sci. Rep. 7 , 4394 (2017).
Turcot, V. et al. Protein-altering variants associated with body mass index implicate pathways that control energy intake and expenditure in obesity. Nat. Genet. 50 , 26–41 (2018). This large-scale exome-wide discovery study reports the use of array data to identify rare coding variations associated with BMI .
Emdin, C. A. et al. Analysis of predicted loss-of-function variants in UK Biobank identifies variants protective for disease. Nat. Commun. 9 , 1613 (2018).
Akbari, P. et al. Sequencing of 640,000 exomes identifies GPR75 variants associated with protection from obesity. Science 373 , eabf8683 (2021). This large-scale exome-wide discovery study reports the use of WES data to identify mutations associated with BMI .
Baggio, L. L. & Drucker, D. J. Biology of incretins: GLP-1 and GIP. Gastroenterology 132 , 2131–2157 (2007).
Antolin-Fontes, B. et al. The habenular G-protein-coupled receptor 151 regulates synaptic plasticity and nicotine intake. Proc. Natl Acad. Sci. USA 117 , 5502–5509 (2020).
Winkler, T. W. et al. The influence of age and sex on genetic associations with adult body size and shape: a large-scale genome-wide interaction study. PLoS Genet. 11 , e1005378 (2015).
Graff, M. et al. Genome-wide physical activity interactions in adiposity — a meta-analysis of 200,452 adults. PLoS Genet. 13 , e1006528 (2017).
Smith, C. E. et al. Genome-wide interactions with dairy intake for body mass index in adults of European descent. Mol. Nutr. Food Res. 62 , 1700347 (2018).
Justice, A. E. et al. Genome-wide meta-analysis of 241,258 adults accounting for smoking behaviour identifies novel loci for obesity traits. Nat. Commun. 8 , 14977 (2017).
Qi, Q. et al. Fried food consumption, genetic risk, and body mass index: gene–diet interaction analysis in three US cohort studies. BMJ 348 , g1610 (2014).
Kilpelainen, T. O. et al. Physical activity attenuates the influence of FTO variants on obesity risk: a meta-analysis of 218,166 adults and 19,268 children. PLoS Med. 8 , e1001116 (2011).
Yeo, G. S. H. Genetics of obesity: can an old dog teach us new tricks? Diabetologia 60 , 778–783 (2017).
Locke, A. E. et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 518 , 197–206 (2015). This large-scale GWAS for BMI shows that BMI-associated loci frequently localize in or near genes that act in the brain .
Friedman, J. M. & Halaas, J. L. Leptin and the regulation of body weight in mammals. Nature 395 , 763–770 (1998).
Ahima, R. S. et al. Role of leptin in the neuroendocrine response to fasting. Nature 382 , 250–252 (1996).
Cowley, M. A. et al. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron 24 , 155–163 (1999).
Bertagna, X. Proopiomelanocortin-derived peptides. Endocrinol. Metab. Clin. North Am. 23 , 467–485 (1994).
Ollmann, M. M. et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 278 , 135–138 (1997).
Butler, A. A. et al. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology 141 , 3518–3521 (2000).
Chen, A. S. et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat. Genet. 26 , 97–102 (2000).
Doche, M. E. et al. Human SH2B1 mutations are associated with maladaptive behaviors and obesity. J. Clin. Invest. 122 , 4732–4736 (2012).
Marenne, G. et al. Exome sequencing identifies genes and gene sets contributing to severe childhood obesity, linking PHIP variants to repressed POMC transcription. Cell Metab. 31 , 1107–1119.e12 (2020).
Asai, M. et al. Loss of function of the melanocortin 2 receptor accessory protein 2 is associated with mammalian obesity. Science 341 , 275–278 (2013).
Michaud, J. L. et al. Sim1 haploinsufficiency causes hyperphagia, obesity and reduction of the paraventricular nucleus of the hypothalamus. Hum. Mol. Genet. 10 , 1465–1473 (2001).
Farooqi, I. S. et al. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N. Engl. J. Med. 348 , 1085–1095 (2003).
Wade, K. H. et al. Loss-of-function mutations in the melanocortin 4 receptor in a UK birth cohort. Nat. Med. 27 , 1088–1096 (2021). This paper establishes the frequency of loss-of-function mutations in MC4R to be 0.3%, much more common than previously appreciated .
van der Klaauw, A. et al. Role of melanocortin signalling in the preference for dietary macronutrients in human beings. Lancet 385 , S12 (2015).
Raffan, E. et al. A deletion in the canine POMC gene is associated with weight and appetite in obesity-prone Labrador retriever dogs. Cell Metab. 23 , 893–900 (2016).
Kim, K. S., Larsen, N., Short, T., Plastow, G. & Rothschild, M. F. A missense variant of the porcine melanocortin-4 receptor (MC4R) gene is associated with fatness, growth, and feed intake traits. Mamm. Genome 11 , 131–135 (2000).
Aspiras, A. C., Rohner, N., Martineau, B., Borowsky, R. L. & Tabin, C. J. Melanocortin 4 receptor mutations contribute to the adaptation of cavefish to nutrient-poor conditions. Proc. Natl Acad. Sci. USA 112 , 9668–9673 (2015).
Pinto, S. et al. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science 304 , 110–115 (2004).
Davies, A. M. The role of neurotrophins in the developing nervous system. J. Neurobiol. 25 , 1334–1348 (1994).
McAllister, A. K., Katz, L. C. & Lo, D. C. Opposing roles for endogenous BDNF and NT-3 in regulating cortical dendritic growth. Neuron 18 , 767–778 (1997).
Korte, M. et al. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc. Natl Acad. Sci. USA 92 , 8856–8860 (1995).
Kernie, S. G., Liebl, D. J. & Parada, L. F. BDNF regulates eating behavior and locomotor activity in mice. EMBO J. 19 , 1290–1300 (2000).
Xu, B. et al. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat. Neurosci. 6 , 736–742 (2003).
Xu, B. & Xie, X. Neurotrophic factor control of satiety and body weight. Nat. Rev. Neurosci. 17 , 282–292 (2016).
Rios, M. et al. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol. Endocrinol. 15 , 1748–1757 (2001).
Gray, J. et al. Hyperphagia, severe obesity, impaired cognitive function, and hyperactivity associated with functional loss of one copy of the brain-derived neurotrophic factor (BDNF) gene. Diabetes 55 , 3366–3371 (2006).
Yeo, G. S. et al. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nat. Neurosci. 7 , 1187–1189 (2004).
Thorleifsson, G. et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat. Genet. 41 , 18–24 (2009).
Finucane, H. K. et al. Heritability enrichment of specifically expressed genes identifies disease-relevant tissues and cell types. Nat. Genet. 50 , 621–629 (2018).
Ndiaye, F. K. et al. The expression of genes in top obesity-associated loci is enriched in insula and substantia nigra brain regions involved in addiction and reward. Int. J. Obes. 44 , 539–543 (2020).
Loos, R. J. & Yeo, G. S. The bigger picture of FTO: the first GWAS-identified obesity gene. Nat. Rev. Endocrinol. 10 , 51–61 (2014).
Church, C. et al. Overexpression of Fto leads to increased food intake and results in obesity. Nat. Genet. 42 , 1086–1092 (2010).
Fischer, J. et al. Inactivation of the Fto gene protects from obesity. Nature 458 , 894–898 (2009).
Cheung, M. K., Gulati, P., O’Rahilly, S. & Yeo, G. S. FTO expression is regulated by availability of essential amino acids. Int. J. Obes. 37 , 744–747 (2013).
Gulati, P. et al. Role for the obesity-related FTO gene in the cellular sensing of amino acids. Proc. Natl Acad. Sci. USA 110 , 2557–2562 (2013).
Karra, E. et al. A link between FTO, ghrelin, and impaired brain food-cue responsivity. J. Clin. Invest. 123 , 3539–3551 (2013).
Hess, M. E. et al. The fat mass and obesity associated gene (Fto) regulates activity of the dopaminergic midbrain circuitry. Nat. Neurosci. 16 , 1042–1048 (2013).
Ruud, J. et al. The fat mass and obesity-associated protein (FTO) regulates locomotor responses to novelty via D2R medium spiny neurons. Cell Rep. 27 , 3182–3198.e9 (2019).
Stratigopoulos, G., LeDuc, C. A., Cremona, M. L., Chung, W. K. & Leibel, R. L. Cut-like homeobox 1 (CUX1) regulates expression of the fat mass and obesity-associated and retinitis pigmentosa GTPase regulator-interacting protein-1-like (RPGRIP1L) genes and coordinates leptin receptor signaling. J. Biol. Chem. 286 , 2155–2170 (2011).
Stratigopoulos, G. et al. Hypomorphism for RPGRIP1L, a ciliary gene vicinal to the FTO locus, causes increased adiposity in mice. Cell Metab. 19 , 767–779 (2014).
Stratigopoulos, G. et al. Hypomorphism of Fto and Rpgrip1l causes obesity in mice. J. Clin. Invest. 126 , 1897–1910 (2016).
Smemo, S. et al. Obesity-associated variants within FTO form long-range functional connections with IRX3. Nature 507 , 371–375 (2014).
Claussnitzer, M. et al. FTO obesity variant circuitry and adipocyte browning in humans. N. Engl. J. Med. 373 , 895–907 (2015).
O’Rahilly, S., Coll, A. P. & Yeo, G. S. FTO obesity variant and adipocyte browning in humans. N. Engl. J. Med. 374 , 191 (2016).
PubMed Google Scholar
Sobreira, D. R. et al. Extensive pleiotropism and allelic heterogeneity mediate metabolic effects of IRX3 and IRX5. Science 372 , 1085–1091 (2021).
Almen, M. S. et al. The obesity gene, TMEM18, is of ancient origin, found in majority of neuronal cells in all major brain regions and associated with obesity in severely obese children. BMC Med. Genet. 11 , 58 (2010).
Larder, R. et al. Obesity-associated gene TMEM18 has a role in the central control of appetite and body weight regulation. Proc. Natl Acad. Sci. USA 114 , 9421–9426 (2017). Aside from the efforts at characterizing the FTO locus, this paper establishes a plausible role for yet another GWAS-identified candidate gene, TMEM18 , in energy homeostasis .
Wiemerslage, L. et al. The Drosophila ortholog of TMEM18 regulates insulin and glucagon-like signaling. J. Endocrinol. 229 , 233–243 (2016).
Biederer, T. et al. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science 297 , 1525–1531 (2002).
Yan, X. et al. Cadm2 regulates body weight and energy homeostasis in mice. Mol. Metab. 8 , 180–188 (2018).
Rathjen, T. et al. Regulation of body weight and energy homeostasis by neuronal cell adhesion molecule 1. Nat. Neurosci. 20 , 1096–1103 (2017).
Wheeler, E. et al. Genome-wide SNP and CNV analysis identifies common and low-frequency variants associated with severe early-onset obesity. Nat. Genet. 45 , 513–517 (2013).
Schafer, M., Brauer, A. U., Savaskan, N. E., Rathjen, F. G. & Brummendorf, T. Neurotractin/kilon promotes neurite outgrowth and is expressed on reactive astrocytes after entorhinal cortex lesion. Mol. Cell. Neurosci. 29 , 580–590 (2005).
Matikainen-Ankney, B. A. & Kravitz, A. V. Persistent effects of obesity: a neuroplasticity hypothesis. Ann. N. Y. Acad. Sci. 1428 , 221–239 (2018).
Lee, A. W. et al. Functional inactivation of the genome-wide association study obesity gene neuronal growth regulator 1 in mice causes a body mass phenotype. PLoS ONE 7 , e41537 (2012).
Joo, Y., Kim, H., Lee, S. & Lee, S. Neuronal growth regulator 1-deficient mice show increased adiposity and decreased muscle mass. Int. J. Obes. 43 , 1769–1782 (2019).
Boender, A. J., van Gestel, M. A., Garner, K. M., Luijendijk, M. C. & Adan, R. A. The obesity-associated gene Negr1 regulates aspects of energy balance in rat hypothalamic areas. Physiol. Rep. 2 , e12083 (2014).
Singh, K. et al. Neuronal growth and behavioral alterations in mice deficient for the psychiatric disease-associated negr1 gene. Front. Mol. Neurosci. 11 , 30 (2018).
Szczurkowska, J. et al. NEGR1 and FGFR2 cooperatively regulate cortical development and core behaviours related to autism disorders in mice. Brain 141 , 2772–2794 (2018).
PubMed PubMed Central Google Scholar
Noh, K. et al. Negr1 controls adult hippocampal neurogenesis and affective behaviors. Mol. Psychiatry 24 , 1189–1205 (2019).
Cano-Gamez, E. & Trynka, G. From GWAS to function: using functional genomics to identify the mechanisms underlying complex diseases. Front. Genet. 11 , 424 (2020).
Loos, R. J. et al. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat. Genet. 40 , 768–775 (2008).
Chambers, J. C. et al. Common genetic variation near MC4R is associated with waist circumference and insulin resistance. Nat. Genet. 40 , 716–718 (2008).
Yaghootkar, H. et al. Genetic studies of leptin concentrations implicate leptin in the regulation of early adiposity. Diabetes 69 , 2806–2818 (2020).
Kichaev, G. et al. Leveraging polygenic functional enrichment to improve GWAS power. Am. J. Hum. Genet. 104 , 65–75 (2019).
Pulit, S. L. et al. Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum. Mol. Genet. 28 , 166–174 (2019).
Wang, Y. et al. Melanocortin 4 receptor signals at the neuronal primary cilium to control food intake and body weight. J. Clin. Invest. 131 , e142064 (2021).
Article CAS PubMed Central Google Scholar
Farooqi, I. S. et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N. Engl. J. Med. 341 , 879–884 (1999). This paper is the first description of the treatment of leptin deficiency using leptin replacement therapy .
Farooqi, I. S. & O’Rahilly, S. 20 years of leptin: human disorders of leptin action. J. Endocrinol. 223 , T63–T70 (2014).
Yeo, G. S. H. et al. The melanocortin pathway and energy homeostasis: from discovery to obesity therapy. Mol. Metab. 48 , 101206 (2021).
Kuhnen, P. et al. Proopiomelanocortin deficiency treated with a melanocortin-4 receptor agonist. N. Engl. J. Med. 375 , 240–246 (2016). This paper is the first to demonstrate that the melanocortin pathway is a relevant and, so far, safe therapeutic target for the treatment of at least rare genetic causes of obesity .
Clement, K. et al. MC4R agonism promotes durable weight loss in patients with leptin receptor deficiency. Nat. Med. 24 , 551–555 (2018).
Clement, K. et al. Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: single-arm, open-label, multicentre, phase 3 trials. Lancet Diabetes Endocrinol. 8 , 960–970 (2020).
Ayers, K. L. et al. Melanocortin 4 receptor pathway dysfunction in obesity: patient stratification aimed at MC4R agonist treatment. J. Clin. Endocrinol. Metab. 103 , 2601–2612 (2018).
Heymsfield, S. B. et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA 282 , 1568–1575 (1999).
Collet, T. H. et al. Evaluation of a melanocortin-4 receptor (MC4R) agonist (setmelanotide) in MC4R deficiency. Mol. Metab. 6 , 1321–1329 (2017).
Khera, A. V. et al. Polygenic prediction of weight and obesity trajectories from birth to adulthood. Cell 177 , 587–596.e9 (2019). This study describes a comprehensive PGS and its association with BMI throughout the life course .
Loos, R. J. F. 15 years of genome-wide association studies and no signs of slowing down. Nat. Commun. 11 , 5900 (2020).
Pearce, L. R. et al. KSR2 mutations are associated with obesity, insulin resistance, and impaired cellular fuel oxidation. Cell 155 , 765–777 (2013).
Greenfield, J. R. et al. Modulation of blood pressure by central melanocortinergic pathways. N. Engl. J. Med. 360 , 44–52 (2009).
Torkamani, A. & Topol, E. Polygenic risk scores expand to obesity. Cell 177 , 518–520 (2019).
Download references
Acknowledgements
R.J.F.L. is supported by funding from Novo Nordisk Foundation (NNF Laureate Award) and the US National Institutes of Health (R01DK110113; R01DK107786; R01HL142302; R01 DK124097). G.S.H.Y. is supported by the Medical Research Council (MRC Metabolic Diseases Unit (MC_UU_00014/1)). The authors thank M. Guindo Martinez for her help with creating data for Fig. 3 and Supplementary Tables 1 and 2.
Author information
Authors and affiliations.
Novo Nordisk Foundation Center for Basic Metabolic Research, University of Copenhagen, Copenhagen, Denmark
Ruth J. F. Loos
Charles Bronfman Institute for Personalized Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, USA
Mindich Child Health and Development Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA
Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA
MRC Metabolic Diseases Unit, University of Cambridge Metabolic Research Laboratories, Wellcome-MRC Institute of Metabolic Science, Addenbrooke’s Hospital, Cambridge, UK
Giles S. H. Yeo
You can also search for this author in PubMed Google Scholar
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Correspondence to Ruth J. F. Loos or Giles S. H. Yeo .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Peer review information.
Nature Reviews Genetics thanks D. Meyre, T. Fall and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
23andMe: https://research.23andme.com/collaborate/
All of Us: https://allofus.nih.gov/
Biobank Japan: http://jenger.riken.jp/en/
Genetic Investigation for ANthropometric Traits (GIANT) consortium: https://portals.broadinstitute.org/collaboration/giant/index.php/Main_Page
Million Veterans Project: https://www.research.va.gov/mvp/
NCD Risk Factor Collaboration (NCD RisC): https://www.ncdrisc.org/data-visualisations.html
UK Biobank: https://www.ukbiobank.ac.uk/
Supplementary information
Supplementary information.
An environment that promotes weight gain.
A severe, early-onset form of obesity, caused by a single-gene mutation, with little or no influence of the environment.
A common multifactorial form of obesity, resulting from an interaction between the obesogenic environment and hundreds of genetic variants.
An approach used to understand the function of a gene by analysing the consequences of genetically manipulating specific sequences within the gene.
A hypothesis-driven approach to study the effect of a given gene (chosen based on the current understanding of its biology and pathophysiology) on susceptibility to the phenotype under study.
A method that relies on the relatedness of study participants to test whether certain chromosomal regions co-segregate with a disease or trait across generations.
(GWAS). A hypothesis-generating approach that screens whole genomes for associations between genetic variants and a phenotype of interest at much higher resolution than is possible for genome-wide linkage studies, and is thus better able to narrow down the associated locus.
(PGS). A measure used to assess an individual’s genetic susceptibility to disease, calculated by summing the number of disease-increasing alleles, weighted by each variant’s effect size observed in a genome-wide association study.
(AUC ROC ). A metric used to assess the ability of a predictor to discriminate between individuals with and without a disease. The AUC ranges from 0.50 (equal to tossing a coin) to 1.0 (perfect prediction).
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Loos, R.J.F., Yeo, G.S.H. The genetics of obesity: from discovery to biology. Nat Rev Genet 23 , 120–133 (2022). https://doi.org/10.1038/s41576-021-00414-z
Download citation
Accepted : 17 August 2021
Published : 23 September 2021
Issue Date : February 2022
DOI : https://doi.org/10.1038/s41576-021-00414-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Genetic effects of sequence-conserved enhancer-like elements on human complex traits.
- Wing Hung Wong
Genome Biology (2024)
A low-calorie meal replacement improves body composition and metabolic parameters in shift workers with overweight and obesity: a randomized, controlled, parallel group trial
- Piumika Sooriyaarachchi
- Ranil Jayawardena
- Neil A. King
Nutrition & Metabolism (2024)
Hyperphagia and impulsivity: use of self-administered Dykens’ and in-house impulsivity questionnaires to characterize eating behaviors in children with severe and early-onset obesity
- Lara Arnouk
- Hélène Chantereau
- Béatrice Dubern
Orphanet Journal of Rare Diseases (2024)
Associations between maternal pre-pregnancy BMI and infant striatal mean diffusivity
- Aylin Rosberg
- Harri Merisaari
- Jetro J. Tuulari
BMC Medicine (2024)
Identifying latent genetic interactions in genome-wide association studies using multiple traits
- Andrew J. Bass
- Shijia Bian
- Michael P. Epstein
Genome Medicine (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.

IMAGES
COMMENTS
The combined burden of underweight and obesity has increased in most countries, driven by an increase in obesity, while underweight and thinness remain prevalent in south Asia and parts of Africa. A healthy nutrition transition that enhances access to nutritious foods is needed to address the remaining burden of underweight while curbing and reversing the increase in obesity.
However, this SLR also reveals that only limited research has reported using ML specifically for detecting obesity. This aspect remains a promising research area because ML techniques can provide far more robust rates of prediction accuracy than those achieved using simple techniques, including statistical methods, such as linear regression.
In the United States, overweight and obesity are chronic diseases that contribute to excess morbidity and mortality. Despite public health efforts, these disorders are on the rise, and their consequences are burgeoning. 1 The Centers for Disease Control and Prevention report that during 2017 to 2018, the prevalence of obesity in the United States was 42.4%, which was increased from the ...
There has been a significant global increase in obesity rate during the last 50 years. Obesity is defined as when a person has a body mass index [BMI (kg/m 2), dividing a person's weight by the square of their height] greater than or equal to 30, overweight is defined as a BMI of 25.0-29.9. Being overweight or obesity is linked with more ...
Our findings for global obesity trends indicate that obesity prevalence has increased in the last few decades across the world. The GBD study showed that between 1980 and 2015 obesity prevalence doubled in 73 countries and the NCD-RisC [ 16 , 17 ] found that age-standardized prevalence of obesity increased from 3.2% to 10.8% in men and from 6.4 ...
Obesity is a chronic and multifactorial disease and has become a worldwide epidemic with devastating health and economic consequences. In 2015, there were an estimated 603.7 million adults and 107.7 million children with obesity worldwide, and the rate of obesity had doubled in nearly half of countries since 1980. 1 Projections estimate that obesity will affect about 1 in every 2 adults in the ...
Given childhood obesity rates, research has lately focused on the role of obesity in early life and subsequent adulthood disease. Obesity in childhood or adolescence has been associated with twofold or higher risk of adult hypertension, coronary heart disease, and stroke . A recent study pooling data from four child cohorts (aged 11 years at ...
New study released by the Lancet shows that, in 2022, more than 1 billion people in the world are now living with obesity. Malnutrition, in all its forms, includes undernutrition, inadequate vitamins or minerals, overweight and obesity. Undernutrition is responsible for half of the deaths of children under 5 and obesity can cause noncommunicable diseases such as cardiovascular diseases ...
The prevalence of obesity has tripled over the past four decades, imposing an enormous burden on people's health. ... They highlight the commonalities revealed by recent studies and discuss the ...
Almost 20 years ago, the World Health Organization (WHO) declared the problem of rising levels of obesity a 'global epidemic', 1 yet the prevalence of overweight (body mass index (BMI; a ratio of weight to height commonly used to categorise weight status) ⩾ 25 kg/m 2) and obesity (BMI ⩾ 30 kg/m 2) has continued to rise. 2,3 In 2016, more than 1.9 billion adults (39% of the world's ...