- Earth Science
- Physics & Engineering
- Science Kits
- Microscopes
- Science Curriculum and Kits
- About Home Science Tools
Science Projects > Chemistry Projects > Splitting Water: Electrolysis Experiments + Video

Splitting Water: Electrolysis Experiments + Video
Electrolysis is the process by which an electric current is passed through a substance to affect a chemical change. The chemical change occurs when the substance loses electrons (oxidation) or gains them (reduction). In the two experiments listed below, the first reactive substance is water and the second one is a copper sulfate solution.
Electrolysis is used to remove hair, split compounds, and in the manufacturing process to decorate, strengthen, and make metal surfaces more resistant to rust.
>>Watch this video to see the electroplating of a copper key>>
An Electrolysis of Water Experiment and an Electroplating Experiment
Electrolysis: splitting water.
For this experiment, you can gather your own supplies or buy a complete water electrolysis kit .
Adult supervision required.
What You Need:
- 6-volt or 9-volt battery
- Two alligator clip leads or insulated wire
- Beaker or glass
- Piece of thin cardboard or card stock
- Two #2 pencils
What You Do:
1. Fill the beaker or glass with warm water.
2. Carefully remove the erasers and metal sleeves so you can sharpen both ends of each pencil. These pencils are your electrodes . The graphite in them will conduct electricity, but won’t dissolve into the water.
3. Cut a piece of the cardboard to fit over the beaker, then punch two holes in the center of the cardboard about an inch apart. Push the pencils through the holes and set them in the glass. They should extend into the water, but not touch the bottom of the glass. The cardboard will hold them in place.
4. Connect each pencil to the battery with an alligator clip lead attached to the exposed graphite (pencil lead). If you don’t have alligator clip leads, use two lengths of wire and strip an inch of insulation off each end. Wrap the wire around the graphite of each pencil and connect the wires to the battery. You may need to use tape to hold the wires in place.

What Happened:
As soon as you connect the wires to the battery, you will see bubbles appearing around each of the pencil tips in the water and floating upward. Those bubbles are the components of water—hydrogen and oxygen gas—that have been split apart by the electricity as it travels through the water from one pencil to the other. The pencil attached to the negative terminal of the battery collects hydrogen gas while the one connected to the positive terminal collects oxygen. Does one pencil collect more bubbles than the other? Which one? Why do you think this is?
(Hint: Water’s chemical name is H 2 O because it has two hydrogen atoms to every one oxygen atom .)
Further experimenting:
- Try adding an electrolyte to the water in the beaker. Water doesn’t conduct electricity that well by itself, but any electrolysis of water experiment could be accelerated by adding table salt to the water. When this is done, you should see a change in how quickly the bubbles form.
Safety Note : using salt may produce small amounts of chlorine gas, similar to the amount present when using bleach.
- Try different types of batteries. Can you make electrolysis happen with a 1.5-volt battery? What about if you add an electrolyte?
- With some real electrolysis equipment you can collect the two gases in test tubes to measure the different amounts produced and test their different reactions to a flame.
- For electrolysis to work as true renewable energy, you need to use a clean energy source to run the reaction. Do this electrolysis experiment again using solar cells instead of a battery.
Electroplating: Copper-Plated Key

Adult supervision and chemical safety equipment required.
>> Watch our project video to see this project in action!
- 1.5-volt D battery with battery holder
- Copper sulfate
- Copper electrode (or coil of copper wire)
- Safety equipment
1. Prepare the key for copper-plating by cleaning it with toothpaste or soap and water. Dry it off on a paper towel.
2. Stir copper sulfate into some hot water in a beaker until no more will dissolve. Your solution should be dark blue. Let it cool.
3. Use one alligator clip to attach the copper electrode to the positive terminal of the battery (this is now the anode ) and the other to attach the key to the negative terminal (now called the cathode ).
4. Partially suspend the key in the solution by wrapping the wire lead loosely around a pencil and placing the pencil across the mouth of the beaker. The alligator clip should not touch the solution.
5. Place the copper strip into the solution, making sure it doesn’t touch the key and the solution level is below the alligator clip. An electrical circuit has now formed and current is flowing.
6. Leave the circuit running for 20-30 minutes, or until you are happy with the amount of copper on the key.
The copper sulfate solution is an electrolyte that conducts electricity from one electrode to the other. When the current is flowing, oxidation (loss of electrons) happens at the copper anode, adding copper ions to the solution. Those ions travel on the electric current to the cathode, where reduction (gain of electrons) happens, plating the copper ions onto the key. There were already copper ions present in the copper sulfate solution before you started, but the oxidation reaction at the anode kept replacing them in the solution as they were plated onto the key, keeping the reaction going.
This project has many variables, including the cleanness and smoothness of the key, the strength of the copper sulfate solution, and the strength of the current. If a black soot-like substance starts forming on the key, your solution is not strong enough for the current. Take the electrodes out and add more copper sulfate. When you put them back in, make sure the anode and cathode are as far apart as possible.
There are lots of projects you can do with electroplating! One fun idea is to use a flat piece of brass as your cathode and draw a design on it with an oil-based marker. The copper will not bond where the marker is. After you’re done plating it, you can use acetone (or nail-polish remover) to wipe off the marker, leaving a design of the brass showing through the copper. You can use a little metal polish to make the copper shiny, if you want.
You may want to try this simple copper-plating experiment that doesn’t use electrolysis and requires only household materials.
Renewable Energy Projects:
- Pinwheel Wind Turbine
- Make a Water Wheel
- Build a Solar Oven
- Design & Build a Solar Car
Welcome! Read other Chemistry articles or explore the rest of the Resource Center, which consists of hundreds of free science articles!
Shop for Chemistry Supplies!
Home Science tools offers a wide variety of Chemistry products and kits. Find affordable beakers, test tubes, chemicals, kits, and everything else you need for lab experiments.
Related Articles

Thanksgiving Science Projects eBook
Fun & Easy Science Activities Your Kids Will Love!

How to Plan a Science Fair Event (at your co-op)
There is something about a science fair that brings everyone together, making it the perfect event to host at your homeschool co-op.

10 Science Experiments You Want To Do This Year
Science experiments are one of the most exciting things you can do in your homeschool or classroom! We've put together some thrilling - yet easy - science experiments for you and your kids to do this year. Please remember to keep safety first and protect...

Modeling Ecosystem Food Webs with Owl Pellet Dissection
Grade Range: Adaptable for Grades 3 - 8. Overview: Learn about food webs by dissecting owl pellets. What You Need: Owl Pellet Dissection Kit Activity Objective: Draw and understand a food web based on what is observed in an owl pellet. Safety...

Leaf Chromatography Experiment
To get started with this leaf chromatography experiment, let's first start with the basics of how leaves get their colors. Leaves contain different pigments, which give them their color. Green chlorophyll is the most common type of pigment, but there are also...
JOIN OUR COMMUNITY
Get project ideas and special offers delivered to your inbox.
- PRO Courses Guides New Tech Help Pro Expert Videos About wikiHow Pro Upgrade Sign In
- EDIT Edit this Article
- EXPLORE Tech Help Pro About Us Random Article Quizzes Request a New Article Community Dashboard This Or That Game Forums Popular Categories Arts and Entertainment Artwork Books Movies Computers and Electronics Computers Phone Skills Technology Hacks Health Men's Health Mental Health Women's Health Relationships Dating Love Relationship Issues Hobbies and Crafts Crafts Drawing Games Education & Communication Communication Skills Personal Development Studying Personal Care and Style Fashion Hair Care Personal Hygiene Youth Personal Care School Stuff Dating All Categories Arts and Entertainment Finance and Business Home and Garden Relationship Quizzes Cars & Other Vehicles Food and Entertaining Personal Care and Style Sports and Fitness Computers and Electronics Health Pets and Animals Travel Education & Communication Hobbies and Crafts Philosophy and Religion Work World Family Life Holidays and Traditions Relationships Youth
- Browse Articles
- Learn Something New
- Quizzes Hot
- Happiness Hub
- This Or That Game
- Train Your Brain
- Explore More
- Support wikiHow
- About wikiHow
- Log in / Sign up
- Education and Communications
How to Electrolyse Water
Last Updated: August 26, 2024 Fact Checked
Setting the Experiment Up
Separating the oxygen and hydrogen.
This article was co-authored by Bess Ruff, MA and by wikiHow staff writer, Eric McClure . Bess Ruff is a Geography PhD student at Florida State University. She received her MA in Environmental Science and Management from the University of California, Santa Barbara in 2016. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group. There are 7 references cited in this article, which can be found at the bottom of the page. This article has been fact-checked, ensuring the accuracy of any cited facts and confirming the authority of its sources. This article has been viewed 74,236 times.
Using a small power source and some electrodes, you can separate molecules of water into hydrogen and oxygen. This process is known as electrolysis. On a large scale, electrolysis is used to create hydrogen power, produce alloys, and create chemicals. Today, we’ll show you how to perform an experiment that replicates this process on a smaller scale.
Simple Experiment to Electrolyse Water
Push two metal pins through the bottom of a plastic cup. Fill the cup with salt water and put the pins on a 9-volt battery. One pin will produce hydrogen gas and the other will produce oxygen. Put two test tubes over the pins to see the gases separate.

- Table salt (kosher salt is best since it’s always pure NaCl). You can use baking soda if you don’t have salt.
- A 9-volt battery .
- The metal push pins are easier to use, but we’ll cover both variations. Most tutorials out there use the alligator clips, though.
- Water (distilled water is best, but tap water is fine, too).
- Two glass test tubes .
- A plastic cup .

- Variation: If you’re using alligator clips and spoons, connect the alligator clips to the ends of the spoon so that each spoon has its own cable.

- Aim for about 1-part salt or baking soda to 10-parts water. If you don’t get any bubbles when you start the experiment, add more salt. [4] X Research source

- The reaction is starting! Notice how the spoons or pins start bubbling? They’re beginning to separate the hydrogen and water.
- The spoons cannot touch one another. If they touch, the reaction will stop as you break the circuit.

- The hydrogen and oxygen won’t have a color. You can only see the gas by watching the amount of air in each tube expand. The color of the water will start to turn light brown as this happens.
- How do you know which gas is which? After a few seconds, you’ll notice the gas is filling up in one tube more quickly than the other. The tube with more air is filled with hydrogen. Remember, H20 is two hydrogen and one oxygen, so the tube with twice as much gas is the hydrogen.

- If you don’t add vinegar to the lye, it may damage your sink or the pipes.
- You’ve also produced a tiny amount of chlorine gas during this experiment. Don’t worry though, you haven’t created nearly enough to be dangerous.
Community Q&A
You Might Also Like

- ↑ https://www.education.com/science-fair/article/water-electrolysis/
- ↑ https://youtu.be/HQ9Fhd7P_HA?t=29
- ↑ https://acswebcontent.acs.org/member_communities/Outreach_Activities.pdf
- ↑ https://youtu.be/T-OwWOYHhMI?t=143
- ↑ https://www.energy.gov/sites/prod/files/2014/06/f16/solar_electrolysisofwater.pdf
- ↑ https://youtu.be/HQ9Fhd7P_HA?t=62
- ↑ https://youtu.be/T-OwWOYHhMI?t=277
About This Article

- Send fan mail to authors
Reader Success Stories
Bill Robert
Nov 15, 2022
Did this article help you?

Oct 16, 2016

Featured Articles

Trending Articles

Watch Articles

- Terms of Use
- Privacy Policy
- Do Not Sell or Share My Info
- Not Selling Info
wikiHow Tech Help Pro:
Develop the tech skills you need for work and life
Navigating By Joy
Learning, laughing and loving together, chemistry for kids – how to separate water into hydrogen and oxygen using electrolysis.
We’ve all been told that water is made up of hydrogen and oxygen. But how do we really know that? Can this wet substance that quenches our thirst and cools our bodies on hot summer days really be made up of two gases ?
We tried to separate water into oxygen and hydrogen using electrolysis. We managed it after a series of experiments that left us with even more questions than we had before we started. Which isn’t necessarily a bad thing – curiosity is a great learning state! (See the mysterious case of the missing oxygen, below.)
You can benefit from our mistakes and perform electrolysis the quick way. Here’s how to split water into hydrogen and oxygen using electrolysis. Afterwards I’ll tell you about what we did first, which produced a different gas entirely.
How to separate water into hydrogen and oxygen
What you need.
- glass or plastic tub
- 2 elastic bands
- 2 test tubes (with lids if possible)
- bicarb of soda (1 tbsp)
- graphite pencil leads
- battery (we used 6V, a bit like this one )
- 2 pairs of crocodile clips
- waterproof tape
What you do
See this video for detailed set-up instructions – the elastic band arrangement keeps the test tubes in place perfectly.
If you can’t watch the video, here’s the gist of it: Connect one end of each crocodile clip to a piece of graphite, and the other to the battery. Secure the graphite ends to the bottom of the tub with the graphite sticking up, and place an inverted test tube over each piece of graphite (held in place by the elastic bands). Dissolve the bicarb of soda in the water and fill the tub. Finally, remove each test tube, fill it with the water, and carefully replace it over the graphite. Any gases collected during the electrolysis will replace the water in the tubes, so make sure there are no air bubbles.

What happens
Bubbles of gas quickly start to form at each electrode. More gas collects at the negative electrode (cathode) than at the positive (anode).
How to test your gases
When you’ve collected plenty of gas at each electrode, carefully put the lids on your test tubes (while they’re still underwater).
To test for hydrogen
We hypothesised that the gas at our (negative) cathode was (positively charged) hydrogen. Hydrogen is explosive. It won’t wreck your house in these quantities, but it will make a cool popping noise in the presence of a lighted splinter of wood. You can hear it in the video below.
To test for oxygen
We test for oxygen with a glowing splint. If enough oxygen is present, the splint rekindles. The gas we collected at our anode gave a brief glow which confirmed it to be oxygen, but after the excitement of the popping hydrogen, we were a bit disappointed. We produced much more oxygen later using a different method – see below for a video of our relighting splint.
How does electrolysis work?
Water is a covalent molecule (H20) held together by shared electrons in covalent bonds.
During electrolysis, the molecules are reduced at the cathode to to hydrogen gas, and oxidised at the anode to oxygen gas.
Pure water doesn’t conduct electricity, so we need to add an electrolyte, like bicarbonate of soda. (You wouldn’t believe the number of websites that tell you to use salt. We tried it, and collected a completely different gas. More on that later.)
Twice as much hydrogen as oxygen is produced, reflecting the molecular composition of water.
Here’s a fairly easy-to-follow explanation of the electrolysis of water .
If you’re looking for a more detailed explanation, see Wikipedia .
{Thank you so much, Sarah, for pointing out my earlier misunderstanding and for making this post more accurate!}
The mysterious case of the missing oxygen
(Or, what happens when you use salt as an electrolyte.)
Before we successfully split water into hydrogen and oxygen using the method above, we tried adding salt to help our water conduct electricity. And not just a pinch of salt. I decided that if a little salt would help a bit, then a lot of salt would be even better. (It works for crystals, after all.)
We set up our electrolysis using the same apparatus as above but this time with a saturated salt solution. And there we sat, eagerly looking for our bubbles of hydrogen and oxygen.
What happened? Well, plenty at our cathode. Gas quickly began to fill the test tube. We tested it and discovered it was hydrogen. And at the positive electrode? Not one single bubble of gas! What had happened to the oxygen from our water molecules?
I did a bit of research overnight.
It seems that during the electrolysis of sodium chloride (salt) solution , sodium chloride breaks down at the positive electrode to form chlorine gas and sodium hydroxide solution. (Click the link for a more detailed explanation.) Chlorine dissolves easily in water, so won’t collect as a gas until the solution is saturated and can absorb no more chlorine.
So if our positive electrode was busy attracting chlorine, and hydrogen was collecting at the cathode … what had happened to the oxygen? Or to the sodium from our sodium chloride (NaCl), for that matter? According to the chemists, the sodium and oxygen combine to make sodium hydroxide solution. Further investigation was called for.
We’d left our apparatus set up – disconnected from the battery – overnight. We decided to examine it for clues.
Further investigations
What changes had taken place as a result of electrolysis? Our salt solution had turned a brownish colour. Was this dissolved chlorine? Broken down graphite? Corroded crocodile clip (which had been attached to the anode)?
Filtering the solution . Some of our positive electrode (anode) broke down, leaving black bits in the solution. We use graphite in electrolysis because it is an inert (non-reactive) metal, but perhaps the large amounts of chlorine we produced had caused it to react? We filtered the brown solution to see if any insoluble bits remained. They didn’t. But we did notice some white spots on the filter paper – the chlorine produced at our positive electrode must have bleached the paper!
Testing the pH of the solution We hypothesised that the solution would be slightly alkali due to the sodium hydroxide. But when we tested it, we found the opposite. It was slightly acidic – like chlorine. We guessed this meant the solution must contain more chlorine than hydroxide.
More fun with oxygen
I’m going slightly off topic here, but I promised to say how we created enough oxygen to successfully test for it. We got the idea from going to The Magic of Oxygen show at the Royal Institution. I’d love to share with you one of the demonstrations we saw there.
The presenters asked me if they could borrow a £10 note from me – and then they set fire to it! Here’s a video of my flaming money.
The Magic of Oxygen scientists also demonstrated how to make “elephant toothpaste” by breaking down hydrogen peroxide. We remembered how we once made our own elephant toothpaste . When we got home we decided to make elephant toothpaste again, and use a glowing splint to test for oxygen gas.
When you place a glowing splint into oxygen, the splint re-lights.
Why this is my favourite way to do homeschool science
As you can tell, this was not the the kind of homeschool science demonstration where mum knows exactly what’s going to happen and why. I studied chemistry until I was sixteen – nearly thirty years ago! I didn’t know the answers to many of the questions generated by these experiments.
But not knowing what would happen made me curious and inspired to learn more, and the children were definitely caught up in my excitement. And I’m glad we made the “mistake” of using salt as an electrolyte first, because if we hadn’t we would have missed out on some very cool science!
Have you done any fun science recently?
Have you ever investigated a case of missing oxygen?
I’m appreciatively linking up here:
Weekly Wrap-Up – Weird Unsocialized Homeschoolers Collage Friday – Homegrown Learners The Home Ed Link Up #16 – Adventures in Home Education Science Sunday – Adventures in Mommydom Finishing Strong – Starts at Eight
The Hip Homeschool Hop – Hip Homeschool Moms
82 thoughts on “ Chemistry for kids – How to separate water into hydrogen and oxygen using electrolysis ”
Wow, this is really excellent, so much extension from a seemingly straightforward experiment! We did the very first part of the electrolysis, with slight variation from yours, and didn’t think to test the gas! I really like how you approach science!
Thank you so much, Hwee – you are very kind! I do love it when science unfolds like this did. It feels so easy when one is naturally caught up in the excitement!
You know how I love this kind of science fun!!
Thank you, Phyllis! I love reading about this kind of science fun on your blog, too!
You really are making science fun. These are subjects I figured were too mature/boring for my younger student but this gives me ideas on how to introduce it. I’m visiting from Weekly Wrap Up hop.
Nita, I did wonder if this would go over my kids’ heads but it was something I decided I wanted to do anyway, so any learning on their part was a bonus! Perhaps because of that, they go caught up in the fun and learned quite a bit too!
I love, love, love, love this! It’s awesome.
Thanks Ticia!
Great post! So in depth and yet so easy to understand.
Thank you, Carol. It was one of our favourites so far. As soon as I get hold of a giant bag of M&Ms we’ll be doing your recent science activities – they’ll complement this well!
Excellent experiment! We’ve done a year of fun chemistry but this is definitely going on our must-try list! Thanks for the detailed explanation.
Thanks, Tonia. I’m coming over to chemistry section of your blog for more ideas for fun experiments!
Wow! Will you come teach science to my kids?? This is amazing. I “Pinned” it for later.
LOL – anytime, Leslie – I love this stuff! Plus I just looked up Saint Paul and it looks like my kind of place. 😉 Thank you for the pin, too!
Your enthusiasm for science experiments is evident. I bet you and your kids have so much fun together. Do you enjoy science as much as your children? It certainly seems that way which is probably why your science demonstrations are so successful and mine aren’t!
I do like the look of that elephant toothpaste. Science provides lots of opportunities for interesting photos!
I think I probably do enjoy science at least as much as my children! Once I get around to doing it, that is… It’s so much easier to gather maths books, or pencils and paper to write stories, than it is to set up equipment for science. But once we’ve started, momentum definitely carries us forward. I remember being very inspired by your posts about Charlotte’s interest in chemistry.
It’s funny you mention the elephant toothpaste photos. I enjoyed taking those so much that after we’d finished the experiment, I put the camera memory stick in my pocket so I could look at the photos on my Mac during the children’s swimming lesson. But… when I went to get the memory stick out, it was gone. The only place I could think I might have dropped it was when we’d walked the dog before swimming. So after swimming I went back to the woods … and found the memory stick buried in mud, right where I’d parked the car! The photos on it have been pinned hundreds of times, and they’re near the top of a google image search for “elephant toothpaste.” C suggested I should give all our photos a spell in mud!
I love the way you do science. I need you to teach my guys their science. I keep procrastinating this term because Native Americans are so much more interesting!
Claire – I think you fitted more science into your germ study than I get round to in a year! And C(10) would love to do history at your place!
Nicely done! We’d done something similar a couple years ago – I love your set up! 🙂
Thank you, Eva!
Now that’s some COOL stuff there! We’ve done Elephant Toothpaste before. Kids loved that!
Elephant’s toothpaste is so much fun, isn’t it, Jessy? I must get some more hydrogen peroxide in so we can do it again – it’s such a great science standby!
This is an awesome post! I am pinning for my daughter for this school year. I would love it if you would link up at the Geeky Educational Link Up! http://www.morethanacouponqueen.com/search/label/Geeky%20Educational%20Link%20Up
Thank you so much, Meagan! And what a fabulous sounding link up – I would love to join in, thank you for the invitation!
Thanks for linking up over at the Geeky Educational Link Up. This is a GREAT post!
Thank you so much, Dawnita! And thanks for hosting the link up.
This is just awesome!!! I did this in high school chemistry…but it have never tried it with the kids at home – you’re very brave 🙂 And, all the follow-up!?! Just fantastic.
Thank you so much – you are very sweet!
Brave… foolish …?! I suspect a lot of the science I do with my kids goes over their heads, but they definitely get caught up in my excitement – which can’t be a bad thing, I hope! 😀
This is a good post…you light up some new idea in kids mind ,i am thinking how we can make H2 as a fuel for vehicles and industries….
Thank you! Yes there are lots of applications, aren’t there? We’ve had some interesting discussions stemming from this experiment.
Ok,Welcome,fine i would like to join your group regarding this,i completed my M.sc food science and technology..and now working in an MNC as QA supervisor come analyst..
That sounds like a very interesting course and job!
Yup ,sure its interesting ..me searching jobs in European countries..i heard that there are lots of vacancies… what about your carrier and family..I think you and your group trying to give knowledge to kids..am I right?
Very thanks……. Tell me.
I want to drive a water engine. So my helped.
Thanks for visiting!
Now how do you combine this electrolysis lab with understanding of molecules and atoms?
Hi Danielle, This tied in for us with our “ oxygen pancakes ” activity, in which we looked at the composition of a water molecule and hydrogen and oxygen atoms. (Using food, which always goes down well round here!)
Hello Ms.? or Mr.?
My Name is Daniel and I was wondering how do create oxygen with electrolysis without obtaining chlorine or and black pieces. Pls inform. I put a 9 volt battery in water and i had the same results. Pls help thanks.
Hi Daniel, When we used baking soda (bicarbonate of soda) as our electrolyte we didn’t produce chlorine or black pieces. We only got them when we performed electrolysis on a saturated salt solution. If you follow the procedure outlined at the start of this post you should be able to do the same. I hope this helps. Let me know how you get on.
Hello all.. This is Jewel..I have food technology background.Now I am working in Qatar,from my knowledge what I would like to share you all is ..water is the elixir of life…like our food washing,car washing…water can remove the poisonous material inside our body. The waste removal in our body is in mainly three ways 1.by Sweating 2.by urine 3.by fecal so if we drink more water we can remove maximum waste from our body,while summer time the water mainly goes through the urine and while cold its mainly by urine. So by god gift we can do wonders with water,may be that’s why god given water more to earth. Like fuel burning mechanism,oxygen should need for each process..so by water/oxygen/and good thinking we can do wonders…
Hello Jewel, Thank you for sharing that. Water really is the elixir or life! You’ve inspired me to go grab myself a glass. 🙂
Welcome dear friend..
Sorry While summer its mainly through sweating and less through urine..but cold or Rainy season it’s opposite..
what voltage are you supposed to use?
Thanks, Jesse! We used a 6 volt battery, like this one .
I had used a 9 volt battery. Thank you. I am going to try the elephant toothpaste.
Did the 9 volt battery work, Jesse?
I hope you enjoy elephant toothpaste!
Yes it worked. Why does some greenish foam float on the water?
Anyway the experiment was wonderful.
Hi Jesse, I’m so pleased your electrolysis worked! What did you use as your electrodes, and as your electrolyte? The greenish foam may have been a product of a reaction involving one of them?
Hi, I think this experiment is great, but I just want to point out that your explanation of the electrolysis of water is incorrect. You cited Wikipedia, but you have misinterpreted the information presented there. Water is NOT an ionic substance and the hydrogens and oxygen don’t simply pull apart and collect at the different electrodes. Water is a covalent molecule held together by shared electrons in the covalent chemical bonds. During electrolysis, the molecules are reduced at the cathode to hydrogen gas and oxidized at the anode to oxygen gas. That’s two different reactions going on, not one single splitting. Compounds like table salt are ionic. It dissociate into positive sodium and negative chloride when it dissolves. I recommend that you revisit your Wiki reference and revise your explanation or at least delete it. Try this resource for an easier to understand explanation: http://www.nmsea.org/Curriculum/7_12/electrolysis/electrolysis.htm
Hi Sarah, Thank you so much for taking the time to leave your comment, I appreciate it. I’m going to have a good look at that link and will update the post asap!
You’re welcome, Maqsood.
what to do if we cant get the test tubes
You could try a thin jar? You’d see the gas collecting, though you may struggle to contain it when you try to test it.
Seriously I never wonder we could intoduce chemistry’s conept for kids, but this one is look fun.
Hello madam Please help me. I separete hydrogen from water and make kit To conserve the hydrogen
Thank you for sharing this science experiment. I am using this for my science fair project.
Excellent , I hope your project went well! Thank you for taking the time to comment!
thank you! you saved me from the science fair in my school! i totaly make this Project ! grettings from Honduras!!!(sorry for my bad english)
Fantastic! I hope your project went well, Susana! Greetings to you in Honduras from Brighton, England 🙂
Your English is very good, by the way!
So I think this is a really cool project actually 2 of my kids did a similar project. Kyle my 12 year old boy did a how video games(fortnight) affect aggression you can see it at fortniteburger.net And Chad did a project on if waffles are good for you example blue waffles look more appetizing then regular waffle go to images and look up blue waffles yum yum. #ProudMom #AwesomeKids #LOL
Thanks Debra. your kids’ projects sound really interesting, you so should be proud of your awesome kids !
Is there a video of the whole process when you create the model?
Hi Theo I’m afraid not, we did this several years ago and didn’t think to video the set up. I wish I had!
“We produced much more oxygen later using a different method – see below for a video of our relighting splint.”
What was this other method, please?
Hi Gary, I confess I can’t remember what I was referring to now – we did this a few years ago. But I know we made lots of oxygen from making elephant toothpaste so you could try that. Or one of my children made oxygen using liver and hydrogen peroxide – see the YouTube link below.
http://navigatingbyjoy.com/2013/02/16/elephants-toothpaste-fun-with-catalysts/
https://youtu.be/_Lk9BD0z9zI
this suck! i did not work!!! i did everything! it still did not work. Do not do this!!!
Sorry to hear the experiment didn’t work for you, Jewle. We’ve had some experiences like that too. It’s frustrating when you follow all the instructions but don’t get the result, isn’t it?
Hey Lucinda, thank you, the project was amazing. So i tried this out for myself and i did everything but for oxygen test tube it only got like very little oxygen and then it just stopped and wasn’t working after a few hours i check on it again but it was exactly the same so when decided to remove the test tube all the water got out of it and this yellowish stuff came out. My crocodile clip was also corroded and I was surprised. The hydrogen test tube was fine as nothing happened to it. Can you please help or explain to me why this happened and put me back on the right track? Thank you.
thank you for the article, the information helped me, success always
Hi,i liked your experiment it was vry funto make
Glad to hear that Wendy. Thank you for taking the time to leave a comment!
Hello! Im an 8th grader doing this project for honor science and right now I’ve gotten the whole process going but instead of bubbles in the negative cord it immersed turned brownish and the positive is doing alright and creating bubbles, am I doing something wrong? Please let me know before nov 4 thank you
Sorry I meant immediately turned brown*
I’m near 68 years old, and retited by disabilties, but I do love secince! and doing such experiments are fun still to me! I was a rhvac tech, I did repairs of heating & air condition equipment [residentual]. I remember this making fire from water as an experiment that the science teacher [in junor high did] it was a real attention getter. He told us, [it was called the brown expermint as I recall but that was not the man who first did the experiment it was called that becuse the durring the act of electrolysiss, the water turned brown from the inter action between the voltage applied and the electrolate]. doing this experiment in from of the class…………made him a rock star!! I have always wondered, what are the flue gases coming from burning the h20?? I have asked people who I though might know!, but I nrver did find out. I firgure, that using salt [as the elecyroyate] would put off a chemical by product by product, along with the h20 [hydrogen/oxygen] and anything appling 12 volts dc would do to the end product of conbustion. In closimg I just want to say I hope the young one seeing this experiment done in school get as moved as I did! this was the thing that made me want to know more about stuff. wanting to know more is what made me want to know more about about the device of the world. how does you refrigeratoor keep the milk cold how does the furnace heat the house. how does that gas engine on the lawnmower/lawn tractor work and when you turn a light in a house what actual happens! I must thank all teacher for planting the seads in our younger generation!!! It looks like it 3:30 am, part of the many things is I don’t sleep very good. but yyou just got to do the best with what you have. I keep pushing on because, somebody, somewhere has it worst and God bless them 606
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Notify me of follow-up comments by email.
Notify me of new posts by email.
Your browser is not supported
Sorry but it looks as if your browser is out of date. To get the best experience using our site we recommend that you upgrade or switch browsers.
Find a solution
- Skip to main content
- Skip to navigation

- Back to parent navigation item
- Primary teacher
- Secondary/FE teacher
- Early career or student teacher
- Higher education
- Curriculum support
- Literacy in science teaching
- Periodic table
- Interactive periodic table
- Climate change and sustainability
- Resources shop
- Collections
- Remote teaching support
- Starters for ten
- Screen experiments
- Assessment for learning
- Microscale chemistry
- Faces of chemistry
- Classic chemistry experiments
- Nuffield practical collection
- Anecdotes for chemistry teachers
- On this day in chemistry
- Global experiments
- PhET interactive simulations
- Chemistry vignettes
- Context and problem based learning
- Journal of the month
- Chemistry and art
- Art analysis
- Pigments and colours
- Ancient art: today's technology
- Psychology and art theory
- Art and archaeology
- Artists as chemists
- The physics of restoration and conservation
- Ancient Egyptian art
- Ancient Greek art
- Ancient Roman art
- Classic chemistry demonstrations
- In search of solutions
- In search of more solutions
- Creative problem-solving in chemistry
- Solar spark
- Chemistry for non-specialists
- Health and safety in higher education
- Analytical chemistry introductions
- Exhibition chemistry
- Introductory maths for higher education
- Commercial skills for chemists
- Kitchen chemistry
- Journals how to guides
- Chemistry in health
- Chemistry in sport
- Chemistry in your cupboard
- Chocolate chemistry
- Adnoddau addysgu cemeg Cymraeg
- The chemistry of fireworks
- Festive chemistry
- Education in Chemistry
- Teach Chemistry
- On-demand online
- Live online
- Selected PD articles
- PD for primary teachers
- PD for secondary teachers
- What we offer
- Chartered Science Teacher (CSciTeach)
- Teacher mentoring
- UK Chemistry Olympiad
- Who can enter?
- How does it work?
- Resources and past papers
- Top of the Bench
- Schools' Analyst
- Regional support
- Education coordinators
- RSC Yusuf Hamied Inspirational Science Programme
- Science Education Policy Alliance
- RSC Education News
- Supporting teacher training
- Interest groups

- More navigation items
The electrolysis of solutions
- Five out of five
- No comments
Find out what happens when electricity passes through molten compounds… with water present
When electricity passes through molten compounds, like sodium chloride, the ions move towards the electrode of opposite charge.
This experiment should take 60 minutes.
- Eye protection
- Beaker, 100 cm 3
- Electrodes (S-shaped are preferable)
- Small test tubes for gas collection
- Two crocodile clips
- DC power supply (6 V is reasonable)
- Universal indicator paper
Access to solutions of :
- Sodium chloride 0.5 mol dm –3
- Copper chloride 0.5 mol dm –3
- Potassium iodide 0.5 mol dm –3
- Sodium bromide 0.5 mol dm –3
- Potassium sulfate 0.5 mol dm –3
- Copper(II) sulfate 0.5 mol dm –3
- Silver nitrate 0.1 mol dm –3
Health, safety and technical notes
- Read our standard health and safety guidance .
- Always wear eye protection
- Chlorine is toxic and harmful to the lungs, eyes and respiratory tract. See CLEAPSS Hazcard HC022a .
- Bromine vapour is an irritant and very toxic if inhaled. See CLEAPSS Hazcard HC015b .
- Iodine is harmful by skin contact. See CLEAPSS Hazcard HC045 .
- Hydrogen is extremely flammable. See CLEAPSS Hazcard HC048 .
- Oxygen supports combustion. See CLEAPSS Hazcard HC069 .
- Ensure good ventilation.
- Do not allow chlorine or bromine vapour to be produced for very long.
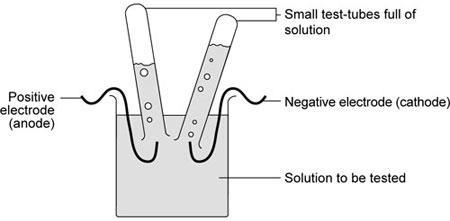
- Set up the apparatus as shown.
- Switch on and observe what happens.
- Try to identify the gases produced (if any).
Teachers may wish to show students how to fill and invert the small test-tubes over the electrodes.
With care, these can be inverted without spilling the liquid. Using small tubes filled with water rather than the test solution is safer.
These are then inverted into the solution to be tested.
The tubes may be clamped or supported by rubber bands wound round the test-tubes and round the electrode.
The class results can be pooled as there will not be time to test all the solutions individually.
Students may need reminding of, or introducing to, the common tests for hydrogen, oxygen, chlorine, bromine and iodine.
- What type of element is formed at the negative electrode?
- What type of element is formed at the positive electrode?
- Your table of results should show some products, which could not come from the compound itself that was electrolysed. Where could these other products have come from?
- Write a general rule for the products formed at (a) the cathode and (b) the anode.
- Metals or hydrogen.
- Non-metals.
- From the water.
- (a) The metal is produced if it is lower than hydrogen in the reactivity series, otherwise hydrogen is produced. (b) Halides give halogens, sulfates and nitrates give oxygen.
The electrolysis of solutions – student sheet
The electrolysis of solutions – teacher notes, additional information.
This practical is part of our Classic chemistry experiments collection.
- 14-16 years
- 16-18 years
- Practical experiments
- Electrochemistry
Specification
- (o) electrolysis of aqueous solutions involving competing ions such as sodium chloride (including electrode equations)
- qualitative tests for ions and organic functional groups
- RP 4: Carry out simple test-tube reactions to identify: cations (Group 2, NH₄⁺), anions (Group 7 [halide ions], OH⁻, CO₃²⁻, SO₄²⁻).
- (ai) qualitative analysis of ions on a test-tube scale; processes and techniques needed to identify the following ions in an unknown compound: anions: CO₃²⁻, by reaction with H⁺(aq) forming CO₂(g)
- (aii) qualitative analysis of ions on a test-tube scale; processes and techniques needed to identify the following ions in an unknown compound: anions: SO₄²⁻, by precipitation with Ba²⁺(aq)
- (aiii) qualitative analysis of ions on a test-tube scale; processes and techniques needed to identify the following ions in an unknown compound: anions: Cl⁻, Br⁻, I⁻
- (aiv) qualitative analysis of ions on a test-tube scale; processes and techniques needed to identify the following ions in an unknown compound: anions: cations: NH₄⁺, by reaction with warm NaOH(aq) forming NH₃
- electrolysis (for extraction of more reactive metals including aluminium)
- Electrolysis is the decomposition of an ionic compound into its elements using electricity.
- Positive ions gain electrons at the negative electrode and negative ions lose electrons at the positive electrode.
- Introduction to oxidation and reduction: simple examples only, e.g. Na with Cl₂, Mg with O₂, Zn with Cu²⁺.
- Oxidising and reducing agents.
- Electrolysis of (i) copper sulfate solution with copper electrodes and (ii) acidified water with inert electrodes.
- Demonstration of electrolysis of aqueous sodium sulfate (using universal indicator) and of aqueous potassium iodide (using phenolphthalein indicator) with inert electrodes. (Half equations only required.)
Related articles

Collision theory and Maxwell–Boltzmann distribution curves
By Dorothy Warren
Use this lesson plan, presentation and student worksheet to recap collision theory, before going on to introduce the Maxwell–Boltzmann distribution.

5 ways to teach reactivity of metals at 14–16
2024-09-04T05:12:00Z By Kristy Turner
Use the importance of metal extraction to help contextualise this topic

Spiral your curriculum for electrochemistry success
2024-08-13T06:05:00Z By Ian McDaid
Discover why electrochemistry doesn’t have to be a challenging topic
No comments yet
Only registered users can comment on this article., more experiments.

‘Gold’ coins on a microscale | 14–16 years
By Dorothy Warren and Sandrine Bouchelkia
Practical experiment where learners produce ‘gold’ coins by electroplating a copper coin with zinc, includes follow-up worksheet

Practical potions microscale | 11–14 years
By Kirsty Patterson Four out of five
Observe chemical changes in this microscale experiment with a spooky twist.

Antibacterial properties of the halogens | 14–18 years
By Kristy Turner
Use this practical to investigate how solutions of the halogens inhibit the growth of bacteria and which is most effective
- Contributors
- Email alerts
Site powered by Webvision Cloud
Science Project Ideas

Electrolysis of Water Experiment
The purpose of the simple experimental setup described here is to demonstrate the electrolysis of water. Proper knowledge on the chemical composition of water will help school children achieve the aim of the project and comprehend the associated theory.
Water Electrolysis (Splitting) Experiment
On passing electricity through water, it splits (or electrolyzes) to give off hydrogen and oxygen gases at the two electrodes.
- Two #2 pencils
- 6 or 9-volt battery
- Two insulated wires or alligator clip leads
- Piece of thin cardstock or cardboard
- Glass or beaker
- Pencil sharpener
- Fill the beaker with warm water.
- Carefully remove the metal sleeves and the erasers of the pencils and sharpen both the ends of each.
[ N.B. The pencils will play the role of the electrodes as the graphite in them conducts electricity but does not dissolve in water.]
- Cut the cardboard piece to a square size that can sit over the mouth of the beaker.
- Punch a couple of holes at its center separating them by about an inch.
- Push the pencils through the holes and set the cardboard above the beaker so that the electrodes do not touch the bottom but remain immersed in the water.
- Use the alligator clips to connect the graphite tip of each pencil to one of the 2 terminals of a battery.
[ N.B. Instead of using the alligator clips you may strip off the insulation at the ends of two pieces of wires and wrap them around the graphite tips and the battery terminals to complete the circuit.]
Note down your observations. As soon as you finish with the connections, you will find bubbles forming around the tips of the pencils immersed in water and moving upwards until they reach the surface.
Electrolysis of Water Experiment Video
Explanation.
It happens because each molecule of water (chemical formula H 2 O) consists of 2 atoms of hydrogen and one of oxygen. When electricity is passed through it by means of the battery, the water gets split or decomposed into its gaseous constituents that are released in the form of bubbles at the electrodes (hydrogen collects at the pencil connected to the negative terminal and oxygen at the positive one). You will watch that the rate of bubbling is more for hydrogen than that of oxygen since the gases maintain a ratio of 2:1 in water respectively.
Water consists of hydrogen and oxygen.
The above fact can be confirmed by inverting small test tubes underwater over the pencils to collect the gases by the downward displacement of water. Now if you introduce a lighted wooden splinter into the gas collected at the cathode (hydrogen), it will make a popping sound. A glowing splinter introduced into the oxygen will, however, rekindle.
Further Research
- Water being a poor conductor of electricity, check if you can speed up the process by adding an electrolyte like a tablespoon of salt or baking soda to the water.
- Repeat the experiment with distilled water instead of the regular water. Does it work?
- Can you make the project more eco-friendly by using solar cells instead of the batteries?
Kids can perform the activity easily at home or in the lab but under the guidance of an adult since the procedure involves electric current though in a very small amount. If done in the lab, attention has to be paid towards creating a proper lab report. When carried out in a science fair, that the experiment serves as a mechanism to extract hydrogen and oxygen from water can also be pointed out.
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.


IMAGES
COMMENTS
An Electrolysis of Water Experiment and an Electroplating Experiment Electrolysis: Splitting Water. For this experiment, you can gather your own supplies or buy a complete water electrolysis kit. Adult supervision required. What You Need: 6-volt or 9-volt battery; Two alligator clip leads or insulated wire; Beaker or glass; Piece of thin ...
Separating hydrogen and oxygen from water is done through electrolysis. To do this experiment, you will need the following items: Table salt (kosher salt is best since it's always pure NaCl). You can use baking soda if you don't have salt. A 9-volt battery. Two metal push pins (or two metal spoons and two alligator clips).
Electrolysis of Water Experiment. Energy is stored in the bonds of molecules. When these bonds split apart, the energy released can be used to do work. ... Your child will do a fun science experiment using soap and pepper flakes to help understand the concepts of water molecules and the surface tension of water. 2nd grade ...
The practical experiment shown in this video is the electrolysis of aqueous copper(II) sulfate. Technician notes and integrated instructions are offered for this experiment. To avoid confusion, electrolysis should be introduced at the basic level by looking at the electrolysis of molten substances, coupling the theory with a video or teacher ...
A fun and easy way to separate water into hydrogen and oxygen using electrolysis. We also tested the gases - hydrogen pops, oxygen rekindles a glowing splint. Navigating By Joy Learning, laughing and loving together ... [residentual]. I remember this making fire from water as an experiment that the science teacher [in junor high did] it was a ...
Anecdotes for chemistry teachers; Literacy in science teaching; More … Climate change and sustainability; Alchemy; On this day in chemistry; Global experiments; PhET interactive simulations; Chemistry vignettes; Context and problem based learning; Journal of the month; Chemistry and art. Back to parent navigation item; Chemistry and art ...
An electrolyzing science project from Science Buddies. Background. To find out what water is made of, it helps to look at its chemical formula, which is H 2 O. This basically tells us that the water molecule is composed of two components: hydrogen (H 2), and oxygen (O 2), or more precisely, two hydrogen atoms and one oxygen atom.Hydrogen and oxygen are gases at room temperature; so, does this ...
The purpose of the simple experimental setup described here is to demonstrate the electrolysis of water. Proper knowledge on the chemical composition of water will help school children achieve the aim of the project and comprehend the associated theory. Water Electrolysis (Splitting) Experiment Hypothesis On passing electricity through water, it splits (or electrolyzes) to give
Electrolysis science fair projects and experiments: topics, ideas, reference resources, and sample projects. Electrolysis ... P=Project E=Experiment. Electrolysis of Water to produce Hydrogen and Oxygen Electrolysis K-12 Experiments & Background Information. Middle School - Grades 7-9.
Electrolysis K-12 experiments & background information for lesson plans, class activities & science fair projects for elementary, middle and high school students. ... In chemistry and manufacturing, electrolysis is a method of using an electric current to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially highly ...