Increasing the value of quality management systems
International Journal of Quality and Service Sciences
ISSN : 1756-669X
Article publication date: 27 July 2021
Issue publication date: 14 September 2021
Over one million organisations have a quality management system (QMS) certified to the ISO 9001 standard; however, the system requires a lot of resources and its value has been questioned. This critique also leads to a questioning of the strategic relevance of quality management. The purpose of this paper is to explore how different types of uses of QMS correlate with management perceptions of quality management in terms of respect, cost and strategic importance.

Design/methodology/approach
The paper is based on a mixed method data collection strategy, quantitative data being collected from a survey in 8 organisations ( n = 108) and qualitative data being collected from 12 interviews with quality managers in 12 different organisations.
The paper shows that a compliance-oriented QMS usage will more likely lead to a view of quality management as costly and of little respect, than a business or improvement-oriented QMS usage. Moreover, it nuances the view on compliance-oriented usage, showing that it is mainly documentation that negatively influences how management views quality management, whereas standardisation that is part of the compliance-oriented use is perceived as more value-adding.
Originality/value
This paper suggests three types of QMS use, namely, business management, improvement, and compliance-oriented use, and that a wise selection of how to use the QMS will affect the respect, strategic importance and cost that management associates with quality management.
- Quality management system
- Quality Management
- Quality audit
Gremyr, I. , Lenning, J. , Elg, M. and Martin, J. (2021), "Increasing the value of quality management systems", International Journal of Quality and Service Sciences , Vol. 13 No. 3, pp. 381-394. https://doi.org/10.1108/IJQSS-10-2020-0170
Emerald Publishing Limited
Copyright © 2021, Ida Gremyr, Jan Lenning, Mattias Elg and Jason Martin.
Published by Emerald Publishing Limited. This article is published under the Creative Commons Attribution (CC BY 4.0) licence. Anyone may reproduce, distribute, translate andcreate derivative works of this article (for both commercial and non-commercial purposes), subject to full attribution to the original publication and authors. The full terms of this licence may be seen at http://creativecommons.org/licences/by/4.0/legalcode
Introduction
Today, more than one million companies and organisations globally are certified in accordance with ISO 9001 ( ISO – International Organization for Standardization, 2018 Survey). In organisations’ quality management work, a substantial amount of time and focus is given to the quality management systems (QMS) ( Elg et al. , 2011 ). Thus, it is important that QMS adds value to the organisations ( Lenning and Gremyr, 2017 ). The interest in QMS has further grown by its potential to support sustainability efforts through integrated management systems, or by improving environmental management systems based on lessons learned from QMS ( Siva et al. , 2016 ). This potential has, however, not yet been fully exploited, and it is suggested that increased formalization and bureaucracy, induced by a certified QMS, is a reason stated for cases in which quality management hinders rather than support implementation of sustainability efforts ( Allur et al. , 2018 ; Barouch and Kleinhans, 2015 ). Even with a focus on QMS per se , that is, not as a support for an environmental management system, QMS has been subject to critique for hindering creativity, being detached from actual practice and providing limited support for quality improvement ( Poksinska et al. , 2006 ), having negative effects on process compliance ( Gray et al. , 2015 ; Karapetrovic et al. , 2010 ) and can limit focus to production and management systems instead of supporting sustainable development and green innovation ( Li et al. , 2018 ).
At the same time, evidence suggests that QMS provides a critical and established structure with potential to create value ( Rönnbäck et al. , 2009 ), contribute to product quality and operational performance ( Iyer et al. , 2013 ; Kafetzopoulos et al. , 2015b ), increase net asset value ( Ochieng et al. , 2015 ) and support continuous improvement ( Lenning and Gremyr, 2017 ). To ensure that the QMS contributes to as much value as possible, it is vital to have support from management and an appreciation of quality management work ( Beer, 2003 ; Dubey et al. , 2018 ; Joiner, 2007 ; Kaynak, 2003 ; Kafetzopoulos et al. , 2015a ; Lakhal et al. , 2006 ), and that management shows and communicates their awareness of the purpose of the QMS ( Zelnik et al. , 2012 ).
This paper aims to contribute to the existing body of research on QMS by describing different ways of using a QMS (drawing on Maguad, 2006 ); detailing and nuancing the understanding of why QMS might be perceived as non-value-adding ( Lenning and Gremyr, 2017 ; Poksinska et al. , 2006 ); and extending research evaluating the impact of QMS beyond a focus on financial performance ( Aba et al. , 2015 ; Cândido et al. , 2016 ). For practitioners, this paper aims to support a broadened understanding of how different usage of a QMS impact managements’ perception of quality management, which in turn possibly impact their willingness to invest resources in QMS.
Drawing on the various ways of operationalizing quality management proposed by ( Maguad, 2006 ), this study investigates three types of QMS usage: QMS as support for developing the quality of an offering; QMS as a tool for daily management; and QMS as a tool for standardization and documentation. The purpose of this paper is to explore how these three different types of uses of QMS correlate with management perceptions of quality management in terms of respect, cost and strategic importance. This study focuses on certified QMS, and a QMS is defined as a part of a management system regarding quality, based upon a set of interconnected or interacting elements of an organization to establish the organisation, operation, policies, objectives and processes to achieve those objectives (ISO 9000, 2015). Thus, such a system of elements can be viewed as a tool and support to reach an organisations’ objectives. In the following section, some background to QMS usage and the three ways of using QMS are provided, after which methods, findings and discussion of the findings are given. Finally, conclusions are drawn.
Theoretical background
Born with the ideas of Deming, Shewart, Juran and Ishikawa nearly four decades ago, quality management has evolved to become an established management philosophy and area of research ( Hackman and Wageman, 1995 ). This philosophy has been presented as being based upon three pillars, namely principles, practices and techniques ( Dean and Bowen, 1994 ). The principles are given as customer focus, continuous improvement and teamwork.
The ISO 9001 management system standard, being a common basis for a QMS, has become universal in its application (ISO Survey, 2018), as well as a central theme in quality management research ( Carnerud, 2018 ). ISO 9001 is claimed to have the potential for contributing to quality improvement ( Sousa and Voss, 2002 ) and improved operational performance ( Kaynak, 2003 ; Psomas and Pantouvakis, 2015 ). However, the value and the effect of a QMS is argued to depend on different factors, such as management attitudes and purposes ( Willar et al. , 2015 ), but also on quality management maturity, implementation strategy and people involvement ( Poksinska, 2010 ).
The type of motivation for implementing a QMS is also said to influence the performance of the system. Organisations focusing on real quality improvements and organisational needs achieve higher benefits from their QMS implementation in areas like quality and operational improvement, compared to those organisations that implement and seek certification of their QMS for external motives, for example, image or customer requirements ( Boiral and Amara, 2009 ; del Castillo-Peces et al. , 2018 ; Poksinska et al. , 2002 ; Sampaio et al. , 2009 ). Thus, a QMS implemented based upon external requirements, tends to focus more on compliance and control and less on organisational efficiency ( Alič and Rusjan, 2010 ).
In the following section, three different ways of working with QMS will be outlined. The three ways draw on Maguad (2006) who argued that quality in the 21st century could be categorised based on orientation in three different directions: business management, improvement and compliance. However, it is said that all three orientations must coincide for an organisation to be successful in their quality work ( Maguad, 2006 ).
Quality management systems as a tool for daily management
Maguad (2006) argued that business management-oriented quality demands an integrated deployment of strategy, and attention to critical success factors, including vision of the business, markets, and core processes. It also requires involvement from top management and every employee in continuous improvement efforts ( Maguad, 2006 ). On an overall level, Sadikoglu and Zehir (2010) studied relationships between quality practices and multiple performance measures and revealed that all practices studied – training, employee management, continuous improvement, information and analysis – were significantly and positively correlated with measures of employee performance, innovation performance, and firm performance. For QMS, it has been shown that they have effects not only on effectivity, product and service quality but also on employees and employers, for example, related to health and safety at the workplace ( Levine and Toffel, 2010 ). Furthermore, Levine and Toffel (2010) show that after being certified, firms experienced a growth in both sales and employment considerably quicker compared to firms that were not certified. Thus, the authors argued that management should consider an ISO 9001 certification as valuable.
If QMS is used as a support for managing the organisation, management will likely show respect for quality management and not view it as cost-driving but rather as being of strategic importance.
Quality management systems as a support for developing the quality of the offering
An improvement-oriented view of quality promotes an integrated approach for process improvement, involves the whole organisation, and has a wide range of applications, such as on service and support operations ( Maguad, 2006 ). In a study of service employees who interact with customers, Coo and Verma (2002) found that the employee’s perceptions of the implemented QMS had an impact on service quality of the actual offering, in terms of reliability, responsiveness, assurance, empathy and tangibles ( Parasuraman et al. , 1988 ), and in turn of the firm’s performance. Coo and Verma (2002) further believe that one success factor of these perceptions were strong leaders who were involved in promoting quality management.
If QMS is seen as supportive of the development of the quality of the organisation’s offering, management will likely show respect for quality management, not viewing it as cost-driving but rather as being of strategic importance.
Quality management systems as a tool for documentation and standardization
A focus on providing documentation, developing procedures and ensuring consistency is said to result in a compliance-oriented approach to quality management ( Maguad, 2006 ). Implementing a QMS standard like ISO 9000 drives standardization. How standardization impacts an organisation can depend on three variables: what is standardized, how the implementation is done, and to what extent activities and processes are standardized ( Poksinska, 2007 ). First, if there is a low motivation for implementing a QMS, it is shown to result in that organization only fulfil the minimum requirements of the ISO 9000. Fulfilling only the minimum requirements may result in the implementation of a QMS that focuses only on describing the existing work practices – that is, standardizing present practices instead of practising the standard ( Poksinska , 2007, 2010 ). Second, if the result of a standardization is positive or negative is also affected by how the standard is implemented. Thus, if the standardization is done with employee involvement (enabling), supporting changes to deficient practices, or if the standard is implemented top-down (coercive), where management wants to discipline work ( Poksinska, 2007 ). Finally, the level of standardization needs to be right, as too high a level of standardization will reduce employees’ work motivation ( Poksinska (2007) .
If QMS is used as a tool for documentation and standardization, management will likely show little respect for quality management and view quality management as cost-driving and lacking in strategic importance.
Methodology
Research instrument
The study was based on a concurrent mixed method data collection strategy ( Creswell et al. , 2007 ) using both quantitative and qualitative data. Quantitative data were gathered using a survey instrument, developed through a literature review, input from senior practitioners, as well as researchers, and input from previously validated questionnaires. Specifically, this paper draws on a set of items focusing on the main function of the QMS ( Poksinska et al. , 2006 ) and management’s perceptions of quality ( Elg et al. , 2011 ) ( Table 1 ).
How would you describe the main role or purpose of the QMS?
How is the QMS used in your organisation?
How do you think management view/perceive the QMS?
For the survey, respondents from eight large-sized Swedish organisations (>1000 employees each) participated in the study (see Table 2 ). Each participating organisation identified 30–50 respondents on different hierarchical levels. The respondents within each organisation were chosen from employees who had dedicated time and responsibility for quality work. The total number of responses was 249 (response rate = 81%), the number of respondents per organisation ranged from 16 to 51. For this paper, the subset of questions used in the analysis focused on management perceptions of quality management and the overall view of the QMS. These questions were only asked of respondents with management responsibilities and resulted in a subset of 108 respondents.
For the interviews, the interviewee sample consisted of twelve quality managers (IP 1–12) with dedicated time and responsibility for quality work. Sample selection was based on organisations offering both products and services, and having established quality management work structures. The sampled organisations covered the following industries: forestry industry, equipment manufacturers, electronics industry, mechanical industry, med-tech industry, logistics industry, and aviation engineering. The interviewees in these organisations focused both product and service quality. Selection was also based on each interviewee having broad areas of responsibility for quality work and also unmediated access to higher management levels, thereby ensuring a relevant knowledge base concerning management perceptions of quality management in general, and the QMS in particular.
Data collection
The survey was administered by e-mail, including a customized invitation letter for each organization and a link to the survey (using the Web-based tool SurveyMonkey). The survey was open for one month per organization, including two rounds of reminders. The interviews were recorded and then transcribed verbatim.
Data analysis
Since the analysed statements in the quantitative data are jointly exhaustive, answers for which no alternative was chosen were considered to be missing values. After excluding rows containing missing values, 108 of the original 249 observations remained. Of these, nine had rows containing the answer “no opinion”. Since this answer cannot be interpreted as an ordinal value, these observations were excluded as well, resulting in a sample of 99 observations. Spearman’s rank correlation coefficient was used to evaluate the monotonic relationships between the ordinal variables. To depend the understanding of the correlations, the mixed method design was exploited as qualitative interview data was used to further the comprehension of the correlations. Hence, focus was on understanding the relevance and meaning of the correlations.
For the analysis of the qualitative data, the transcriptions of the interviews were uploaded into the QSR NVivo 12 software program. A coding scheme was devised using the theory of grounded propositions (see above). The interviews were then subjected to a thematic text analysis using a deductive cross-case analysis strategy ( Miles and Huberman, 1994 ). Data analysis was done by first reading through all the interviews. By using the theoretically derived coding scheme, coding can be described as influenced by the theoretical underpinnings of the propositions and as descriptive by “attributing a class of phenomena to a segment of text” ( Miles and Huberman, 1994 , p.57), based on the grounded propositions. The content of the coded data was thematically analysed whereby general similarities (or discrepancies) between the interviewees could be identified. Finally, the thematic content was evaluated against the conceptual and theoretical underpinnings to further understand the data and draw conclusions. An overview of the coding scheme with quotes illustrating how the data analysis was performed is featured in Table 3 . The results per se will be further elaborated on in the findings section.
Each code category was labelled either to signify a positive view – the use of QMS is viewed with respect in daily work, QMS is viewed as cost reducing, and the use QMS is viewed as strategically important – or to signify a negative view – the use of QMS is not viewed with respect in daily work, QMS is viewed as cost increasing and the use of QMS is not viewed as strategically important.
The study took several steps to achieve acceptable research quality, for example all questions in the survey were based on established instruments, and triangulation of data with questionnaire data and interview data was used to corroborate the findings.
On an overall level, the data shows that the respondents to a large extent agree with all the statements regarding the function and use of the QMS in their organisation ( Table 4 ).
It appears that QMS as a “tool to handle documentation”, “tool for standardisation”, and as having a “significant impact on how the organisation works” are the three statements where most respondents to some extent agree and in other words recognise their way of working with QMS. For statements where a group of respondents do not agree at all, the three other statements stand out. The statement for which most respondents do not agree is that QMS is “a tool that supports efficient management of our organisation”, followed by QMS is “a tool that helps us to fulfil our customers’ needs”, and QMS is “a tool for managing our quality work and improve the quality of our products/services”. As QMS and activities related to designing, implementing, and maintaining the system is a large part of what a quality function does, it arguably will influence how managers view quality management overall. Figure 1 shows the correlations between the level of agreement on the statements related to the function of QMS, and management’s view on quality management in terms of respect, cost and strategic importance.
First, P1 ’s focus on a business management-oriented use of QMS relates to two functions of QMS: impact on work and efficient management ( Table 1 ). These two functions of QMS correlate negatively to management viewing quality management as with a lack of respect and as being costly. On the other hand, there is a positive correlation to viewing quality management as being of strategic importance. Hence, the data points in the same directions as outlined in proposition 1. The findings from the interviews partly support proposition 1 in that management views the impact of QMS on efficient management as positive (e.g. IP8, IP10, IP12). For example, IP7 states that: “The current management at […] has a clear quality aware mentality that benefits everybody […] that works with quality”. However, management can also be perceived as showing a “lack of interest to QMS as to the purpose of quality management work” (IP1).
Second, P2 encompasses the statements on QMS as a tool focused on customer needs and a tool impacting product/service quality; these two concepts constitute what this paper refers to as an improvement-oriented use of QMS. In the same way as the statements underlying P1 , the statements of “customer needs” and “product/service quality” correlate positively to management acknowledging the strategic importance of quality management. Moreover, there are negative correlations with quality being viewed with little respect and as a costly activity. Looking at the correlation values, these are largest for the statement regarding “customer needs”, which might depend on a larger variation in the responses. The findings from the interviews are mostly in favour of P2 (e.g. IP4, IP8, IP12). Key customer requirements such as sustainability (IP4), and also the function of collecting customer information and understanding customer needs (IP3) is perceived by the management as being directly facilitated by QMS. As an example, IP12 states that: “Auditing is still a big part, because that’s one way you can tell how you’re adhering to what your customers want”. IP4 described the benefit of QMS supporting organizational success like this: “And we have this in order, it will be a competitive advantage, and it’s coming globally; it’s coming in all areas.” However, there are also perceptions of management only perceiving the use of QMS for improvement as a “tick in the box”. The interviews show various degrees of understanding QMS as a tool for improvement by management levels (e.g. IP2, IP8).
Third and last, P3 refers to a compliance-oriented use of QMS and concerns documentation and standardization. The correlations are small, but the results are mixed as compared to the other two propositions. The statement viewing QMS as a tool for documentation, displays correlations supporting parts of P3 . That is, it positively correlates with little respect for quality management and a view of it as being costly. However, the statement on documentation does not correlate with quality management being seen as strategic. Moving to the other statement on a compliance-oriented QMS use (“standardization”), the correlations do not support P3 . The use of QMS as a tool for standardization negatively correlates with all three views on quality management. It does not appear supportive of a view on quality management as costly, or of it being little respected. However, it does have a negative correlation with quality management being viewed as strategic (as outlined in P3 ). Again, the correlations are small and further investigation is needed. The interview findings related to P3 are somewhat ambiguous. Regarding management perceptions that QMS, primarily used as a tool for documentation, increases both work and costs and also reduces respect, the findings support P3 (e.g. IP1, IP2, IP5, IP6). Concerning perceptions of QMS used as a tool for standardization, statements on QMS as filling regulatory purposes recur (e.g. IP8, IP9, IP11). Standardization is viewed as both an imperative and something that is self-evident and “the right thing to do” (IP8) with references to safety and brand perception in order not to “run into problems” (IP9).
To support improved QMS usage and increase the perceived value added by a QMS, there is a need to move beyond the broad conception of QMS usage and move towards a more detailed analysis. This paper contributes to research on QMS by outlining three different ways of using QMS, rather than studying QMS usage overall. Drawing on Maguad (2006) three types of QMS usage are described as being oriented towards business management, improvement or compliance.
First, the business management-oriented use of QMS is operationalised by QMS “significantly impacting the way an organisation works”, and “is a tool that supports efficient management of an organisation”. As assumed in proposition 1, these functions appear to support that management will likely show respect for quality management and not view it as cost-driving but rather as being of strategic importance. This is in line with previous research by, for example, Bunney and Dale (1997) establishing that deployment of quality initiatives will be more successful if they are perceived as closely connected to – and potentially improving upon – current work practices.
Second, the improvement-oriented use of QMS is based on QMS as “a tool that help us to fulfil our customers’ needs”, and “a tool for managing our quality work and improve the quality of our products/services”. The proposed impact of these functions is supported, thus ensuring respect for quality management and not viewing it as costly but as strategic ( P2 ). Hence, using QMS to fulfil customer needs and improve the quality of the product or service will positively impact management perception of quality management overall. Previous research has shown that improved quality of the product/service will lead to increased customer satisfaction and loyalty ( Honore Petnji Yaya et al. , 2011 ; Parasuraman et al. , 1988 ), and that improved product/service quality is a benefit of QMS ( Psomas and Pantouvakis, 2015 ). Thus, if QMS is used in a way that can be linked to improved quality and customer satisfaction, this will likely impact management perception of the value added by the QMS.
Third, the results are more mixed in relation to P3 that QMS is used as “a tool for documentation” and “standardization”. This would be correlated with management showing little respect for quality management, viewing it as cost-driving, and not viewing it as strategic. As management perception and support is critical for QMS implementation ( Willar et al. , 2015 ), it is critical to minimize the risk with a too strong focus on documentation conveying a view of QMS as bureaucratic ( Allur et al. , 2018 ) rather than a respected and value-adding activity. However, a certification is still of value as a qualifier in certain business relations ( Boiral and Amara, 2009 ; del Castillo-Peces et al. , 2018 ). This might be a reason that the documentation focus does not appear to have the anticipated negative correlation with management viewing quality management as strategic value. Moreover, a standardisation-focussed use of QMS does not appear to reduce respect for quality management nor lead to it being seen as costly. Perhaps this can be linked to Poksinskàs (2007, 2010) notion of practising the standard rather than standardising current practices. In other words, if standardisation is done with an improvement approach rather than one of pure documentation, it will likely be perceived as beneficial. This is also linked to the function of QMS as having “impact on work”, which is classified as a business management-oriented QMS usage. If this is practised and QMS is allowed to impact actual practices, it will likely mean that QMS is used to standardise and at the same time improve existing work practices.
Overall, the findings support literature pointing to challenges of QMS in terms of focus on compliance rather than organisational efficiency ( Alič and Rusjan, 2010 ), and sometimes not being relevant for actual practice ( Poksinska et al. , 2006 ). However, by distinguishing QMS usage in the three orientations presented above, this study indicates that the documentation focus is what might be the cause for many negative perceptions of the value of QMS. On the other hand, many respondents fully agree that QMS is “a tool that helps us to fulfil our customers’ needs”, which has a relatively high correlation with management viewing quality management as strategic. Contrary to the view of limited value from QMS, this paper supports Poksinska (2007) and Lenning and Gremyr (2017) in that there is potential value in QMS, and that this perceived value will increase if QMS usage is mainly business management- and improvement-oriented, although wisely documented and standardised processes are also required to maintain a certified QMS. An important issue highlighted in the interviews is the risk of using QMS as “quality washing” by management. The interviews indicate that there is still a need to further increase knowledge and understanding within higher management levels on the value of QMS.
The data set underlying this paper is limited in size and the correlations established from the quantitative data are small, yet the qualitative data also supports the propositions. To further establish how an organisation should work with QMS to gain as much benefit as possible, more empirical studies on the three orientations (i.e. business management, improvement and compliance oriented) to QMS are suggested.
Conclusions
Based on an extended view of QMS, this paper has elaborated on three types of QMS use: business management, improvement and compliance-oriented use. The purpose was to explore how these three differing types of uses of QMS correlate with management perceptions of quality management in terms of respect, cost, and strategic importance. Overall, the conclusion is that different ways of working with QMS does not only impact the value of QMS per se , rather it also influences management’s respect for and view of quality management. In terms of difference between the three types of QMS usage, there is a correlation between business management- and improvement-oriented uses of QMS with quality management being respected, and viewed as strategic and not cost-driving. Earlier research has suggested a compliance-oriented use of QMS was the reason for many of the negative perceptions of QMS that in turn was suspected to lead to negative views on quality management in general. However, the findings of this study are somewhat contradictory to this and provide a more nuanced picture showing that, in general, compliance-oriented views might not drive negative perceptions and that it is useful to operationalise compliance into documentation and standardisation. It is suggested that a perception of QMS as having limited value is mainly due to a focus on documentation, whereas work on standardization, which is also part of a compliance-oriented QMS, does not carry similar negative implications. In summary, this study highlights how the perceived strategic value of quality management can be increased through a deliberate design, and choice of an organisation’s ways of using QMS.
Correlation matrix
Overview of organisations in the survey
Coding scheme with illustrative examples
Aba , E. , Badar , M. and Hayden , M. ( 2015 ), “ Impact of ISO 9001 certification on firms financial operating performance ”, International Journal of Quality and Reliability Management , Vol. 33 No. 1 , pp. 78 - 89 , available at: https://doi-org.proxy.lib.chalmers.se/10.1108/IJQRM-02-2014-0021
Alič , M. and Rusjan , B. ( 2010 ), “ Contribution of the ISO 9001 internal audit to business performance ”, International Journal of Quality and Reliability Management , Vol. 27 No. 8 , pp. 916 - 937 , available at: https://doi.org/10.11/02656711011075116
Allur , E. , Heras-Saizarbitoria , I. , Boiral , O. and Testa , F. ( 2018 ), “ Quality and environmental management linkage: a review of the literature ”, Sustainability , Vol. 10 No. 11 , p. 4311 , available at: https://doi.org/10.3390/su10114311
Barouch , G. and Kleinhans , S. ( 2015 ), “ Learning from criticisms of quality management ”, International Journal of Quality and Service Sciences , Vol. 7 Nos 2/3 , pp. 201 - 216 ., available at: https://doi.org/10.1108/IJQSS-02-2015-0026
Beer , M. ( 2003 ), “ Why total quality management programs do not persist: the role of management quality and implications for leading a TQM transformation ”, Decision Sciences , Vol. 34 No. 4 , pp. 623 - 642 , available at: https://doi-org/10.1111/j.1540-5414.2003.02640.x
Boiral , O. and Amara , N. ( 2009 ), “ Paradoxes of ISO 9000 performance: a configurational approach ”, Quality Management Journal , Vol. 16 No. 3 , pp. 36 - 60 , available at: https://doi.org/10.1080/10686967.2009.11918240
Bunney , H.S. and Dale , B.G. ( 1997 ), “ The implementation of quality management tools and techniques: a study ”, The TQM Magazine , Vol. 9 No. 3 , pp. 183 - 189 , available at: https://doi-org/10.1108/09544789710168966
Cândido , C.J. , Coelho , L.M. and Peixinho , R.M. ( 2016 ), “ The financial impact of a withdrawn ISO 9001 certificate ”, International Journal of Operations and Production Management , Vol. 36 No. 1 , pp. 23 - 41 , available at: https://doi-org/10.1108/IJOPM-11-2014-0540
Carnerud , D. ( 2018 ), “ 25 Years of quality management research–outlines and trends ”, International Journal of Quality and Reliability Management , Vol. 35 No. 1 , pp. 208 - 231 , available at: https://doi-org/10.1108/IJQRM-01-2017-0013
del Castillo-Peces , C. , Mercado-Idoeta , C. , Prado-Roman , M. and del Castillo-Feito , C. ( 2018 ), “ The influence of motivations and other factors on the results of implementing ISO 9001 standards ”, European Research on Management and Business Economics , Vol. 24 No. 1 , pp. 33 - 41 , available at: https://doi.org/10.1016/j.iedeen.2017.02.002
Coo , L.S. and Verma , R. ( 2002 ), “ Exploring the linkages between quality system, service quality, and performance excellence: Service providers’ perspectives ”, Quality Management Journal , Vol. 9 No. 2 , pp. 44 - 56 , available at: https://doi.org/10.1080/10686967.2002.11919009
Creswell , J.W. , Plano Clark , V.L. , Gutmann , M. and Hanson , W. ( 2007 ), “ Advanced mixed methods research designs ”, in Tashakkori , A. and Teddlie , C. (Eds), Handbook of Mixed Methods in Social and Behavioral Research , Sage , Thousand Oaks, CA , pp. 619 - 637 .
Dean , J.W. and Bowen , D.E. ( 1994 ), “ Management theory and total quality: improving research and practice through theory development ”, Academy of Management Review , Vol. 19 No. 3 , pp. 392 - 418 , available at: https://doi.org/10.5465/AMR.1994.9412271803
Dubey , R. , Gunasekaran , A. , Childe , S.J. , Papadopoulos , T. , Hazen , B.T. and Roubaud , D. ( 2018 ), “ Examining top management commitment to TQM diffusion using institutional and upper echelon theories ”, International Journal of Production Research , Vol. 56 No. 8 , pp. 2988 - 3006 , availabel at: https://doi.org/10.1080/00207543.2017.1394590
Elg , M. , Gremyr , I. , Hellström , A. and Witell , L. ( 2011 ), “ The role of quality managers in contemporary organisations ”, Total Quality Management and Business Excellence , Vol. 22 No. 8 , pp. 795 - 806 , available at: https://doi.org/10.1080/14783363.2011.593899
Gustafsson , R. , Klefsjö , B. , Berggren , E. and Granfors‐Wellemets , U. ( 2001 ), “ Experiences from implementing ISO 9000 in small enterprises – a study of Swedish organisations ”, The TQM Magazine , Vol. 13 No. 4 , pp. 232 - 246 , available at: https://doi.org/10.1108/09544780110366088
Gray , J.V. , Anand , G. and Roth , A.V. ( 2015 ), “ The influence of ISO 9000 certification on process compliance ”, Production and Operations Management , Vol. 24 No. 3 , pp. 369 - 382 , available at: https://doi.org/10.1111/poms.12252
Hackman , J.R. and Wageman , R. ( 1995 ), “ Total quality management: empirical, conceptual, and practical issues ”, Administrative Science Quarterly , Vol. 40 No. 2 , pp. 309 - 342 , available at: https://doi.org/10.2307/2393640
Honore Petnji Yaya , L. , Marimon , F. and Casadesus , M. ( 2011 ), “ Customer’s loyalty and perception of ISO 9001 in online banking ”, Industrial Management and Data Systems , Vol. 111 No. 8 , pp. 1194 - 1213 , available at: https://doi.org/10.1108/02635571111170767
ISO – International Organization for Standardization ( 2018 ), “ ISO survey of certifications to management system standards – full results ”, The ISO Survey , available at: www.iso.org/the-iso-survey.html (accessed 28 November 2020 ).
Iyer , A. , Saranga , H. and Seshadri , S. ( 2013 ), “ Effect of quality management systems and total quality management on productivity before and after: empirical evidence from the indian auto component industry ”, Production and Operations Management , Vol. 22 No. 2 , pp. 283 - 301 , available at: https://doi.org/10.1111/poms.12000
Joiner , T.A. ( 2007 ), “ Total quality management and performance: the role of organization support and co‐worker support ”, International Journal of Quality and Reliability Management , Vol. 24 No. 6 , pp. 617 - 627 , available at: https://doi.org/10.1108/02656710710757808
Kafetzopoulos , D. , Gotzamani , K. and Gkana , V. ( 2015a ), “ Relationship between quality management, innovation and competitiveness. Evidence from Greek companies ”, Journal of Manufacturing Technology Management , Vol. 26 No. 8 , available at: https://doi.org/10.1108/JMTM-02-2015-0007
Kafetzopoulos , D.P. , Psomas , E.L. and Gotzamani , K.D. ( 2015b ), “ The impact of quality management systems on the performance of manufacturing firms ”, International Journal of Quality and Reliability Management , Vol. 32 No. 4 , pp. 381 - 399 , available at: https://doi.org/10.1108/IJQRM-11-2013-0186
Karapetrovic , S. , Fa , M.C. and Saizarbitoria , I.H. ( 2010 ), “ What happened to the ISO 9000 lustre? An eight-year study ”, Total Quality Management and Business Excellence , Vol. 21 No. 3 , pp. 245 - 267 , available at: https://doi.org/10.1080/14783360903553149
Kaynak , H. ( 2003 ), “ The relationship between total quality management practices and their effects on firm performance ”, Journal of Operations Management , Vol. 21 No. 4 , pp. 405 - 435 , available at: https://doi.org/10.1016/S0272-6963(03)00004-4
Lakhal , L. , Pasin , F. and Limam , M. ( 2006 ), “ Quality management practices and their impact on performance ”, International Journal of Quality and Reliability Management , Vol. 23 No. 6 , pp. 625 - 646 , available at: https://doi.org/10.1108/02656710610672461
Lee , C.Y. ( 2004 ), “ Perception and development of total quality management in small manufacturers: an exploratory study in China ”, Journal of Small Business Management , Vol. 42 No. 1 , pp. 102 - 115 , available at: https://doi.org/10.1111/j.1540-627X.2004.00100.x
Lenning , J. and Gremyr , I. ( 2017 ), “ Making internal audits business-relevant ”, Total Quality Management and Business Excellence , Vol. 28 Nos 9/10 , pp. 1106 - 1121 , available at: https://doi.org/10.1080/14783363.2017.1303891
Levine , D.I. and Toffel , M.W. ( 2010 ), “ Quality management and job quality: how the ISO 9001 standard for quality management systems affects employees and employers ”, Management Science , Vol. 56 No. 6 , pp. 978 - 996 , available at: https://doi.org/10.1287/mnsc.1100.1159
Li , D. , Zhao , Y. , Zhang , L. , Chen , X. and Cao , C. ( 2018 ), “ Impact of quality management on green innovation ”, Journal of Cleaner Production , Vol. 170 , pp. 462 - 470 .
Maguad , B.A. ( 2006 ), “ The modern quality movement: Origins, development and trends ”, Total Quality Management and Business Excellence , Vol. 17 No. 2 , pp. 179 - 203 , available at: https://doi.org/10.1080/14783360500450608
Miles , M.B. and Huberman , A.M. ( 1994 ), Qualitative Data Analysis: An Expanded Sourcebook , Sage .
Ochieng , J. , Muturi , D. and Njihia , S.N. ( 2015 ), “ The impact of ISO 9001 implementation on organizational performance in Kenya ”, The TQM Journal , Vol. 27 No. 6 , pp. 761 - 771 , available at: https://doi.org/10.1108/TQM-06-2015-0071
Parasuraman , A. , Zeithaml , V.A. and Berry , L.L. ( 1988 ), “ Servqual: a multiple-item scale for measuring consumer perc ”, Journal of Retailing , Vol. 64 No. 1 , p. 12 .
Poksinska , B. ( 2007 ), “ Does standardization have a negative impact on working conditions? ”, Human Factors and Ergonomics in Manufacturing , Vol. 17 No. 4 , pp. 383 - 394 , available at: https://doi-org/10.1002/hfm.20080
Poksinska , B. ( 2010 ), “ When does ISO 9000 lead to improvements? ”, International Journal of Productivity and Quality Management , Vol. 5 No. 2 , pp. 124 - 136 , available at: http://dx.doi.org/10.1504/IJPQM.2010.030738
Poksinska , B. , Eklund , J.A.E. and Jörn Dahlgaard , J. ( 2006 ), “ ISO 9001:2000 in small organisations: Lost opportunities, benefits and influencing factors ”, International Journal of Quality and Reliability Management , Vol. 23 No. 5 , pp. 490 - 512 , available at: https://doi-org/10.1108/02656710610664578
Poksinska , B. , Jörn Dahlgaard , J. and Antoni , M. ( 2002 ), “ The state of ISO 9000 certification: a study of Swedish organizations ”, The TQM Magazine , Vol. 14 No. 5 , pp. 297 - 306 , available at: https://doi.org/10.1108/09544780210439734
Psomas , E. and Pantouvakis , A. ( 2015 ), “ ISO 9001 overall performance dimensions: an exploratory study ”, The TQM Journal , Vol. 27 No. 5 , pp. 519 - 531 , available at: https://doi.org/10.1108/TQM-04-2014-0037
Rönnbäck , Å. , Witell , L. and Enquist , B. ( 2009 ), “ Quality management systems and value creation ”, International Journal of Quality and Service Sciences , Vol. 1 No. 3 , pp. 241 - 254 , available at: https://doi.org/10.1108/17566690911004186
Sadikoglu , E. and Zehir , C. ( 2010 ), “ Investigating the effects of innovation and employee performance on the relationship between total quality management practices and firm performance: an empirical study of Turkish firms ”, International Journal of Production Economics , Vol. 127 No. 1 , pp. 13 - 26 , available at: https://doi.org/10.1016/j.ijpe.2010.02.013
Sampaio , P. , Saraiva , P. and Guimarães Rodrigues , A. ( 2009 ), “ ISO 9001 certification research: questions, answers and approaches ”, International Journal of Quality and Reliability Management , Vol. 26 No. 1 , pp. 38 - 58 , available at: https://doi.org/10.1108/02656710910924161
Siva , V. , Gremyr , I. , Bergquist , B. , Garvare , R. , Zobel , T. and Isaksson , R. ( 2016 ), “ The support of quality management to sustainable development: a literature review ”, Journal of Cleaner Production , Vol. 138 , pp. 148 - 157 , available at: https://doi.org/10.1016/j.jclepro.2016.01.020
Sousa , R. and Voss , C.A. ( 2002 ), “ Quality management re-visited: a reflective review and agenda for future research ”, Journal of Operations Management , Vol. 20 No. 1 , pp. 91 - 109 , available at: https://doi.org/10.1016/S0272-6963(01)00088-2
Willar , D. , Coffey , V. and Trigunarsyah , B. ( 2015 ), “ Examining the implementation of ISO 9001 in Indonesian construction companies ”, Vol. 27 No. 1 , pp. 94 - 107 , available at: https://doi.org/10.1108/TQM-08-2012-0060
Zelnik , M. , Maletič , M. , Maletič , D. and Gomišček , B. ( 2012 ), “ Quality management systems as a link between management and employees ”, Total Quality Management and Business Excellence , Vol. 23 No. 1 , pp. 45 - 62 , available at: https://doi-org/10.1080/14783363.2011.637781
Acknowledgements
The authors are grateful for the support from the Swedish Quality Management Academy and the organisations participating in this study. Further, we acknowledge financial support from the Production Area of Advance at Chalmers and the HELIX Competence Centre at Linköping University.
Corresponding author
Related articles, all feedback is valuable.
Please share your general feedback
Report an issue or find answers to frequently asked questions
Contact Customer Support

Message placeholder

1 Introduction
2 presentation of the laboratory and its quality policy, 3 implementation of a quality management system: actions undertaken, 4 discussion, analysis and improvements, 5 conclusion.
- List of figures
Research Article
An overview of Quality Management System implementation in a research laboratory
Valérie Molinéro-Demilly 1 * , Abdérafi Charki 2 , Christine Jeoffrion 3 , Barbara Lyonnet 4 , Steve O'Brien 5 and Luc Martin 6
1 Horticulture and Seeds Research Institute (IRHS-MRU 1345), INRA/Agrocampus Ouest/University of Angers-42, rue Georges Morel, 49071 Beucouzé Cedex, France 2 Angevin Research Laboratory in Systems Engineering (LARIS–EA 7315), University of Angers, 62 avenue Notre Dame du Lac, 49000 Angers, France 3 Psychology Laboratory of Pays de la Loire (LPPL-UPRES EA 4638), University of Nantes, BP 81 227, 44312 Nantes cedex 3, France 4 Economy and Management Laboratory (LEMNA), University of Nantes, Chemin de la Censive du Tertre, B.P. 81227, 44312 Nantes Cedex 3, France 5 Decision Support Systems Research Centre (CERADE), ESAIP School of Engineering, 18 rue du 8 mai 1945, 49180 St Barthélemy d'Anjou, France 6 Agricultural Research Centre for International Development (CIRAD), Avenue Agropolis, 34398 Montpellier Cedex 5, France
* Corresponding author: [email protected]
Received: 7 June 2017 Accepted: 11 November 2017
The aim of this paper is to show the advantages of implementing a Quality Management System (QMS) in a research laboratory in order to improve the management of risks specific to research programmes and to increase the reliability of results. This paper also presents experience gained from feedback following the implementation of the Quality process in a research laboratory at INRA, the French National Institute for Agronomic Research and details the various challenges encountered and solutions proposed to help achieve smoother adoption of a QMS process. The 7Ms (Management, Measurement, Manpower, Methods, Materials, Machinery, Mother-nature) methodology based on the Ishikawa ‘Fishbone’ diagram is used to show the effectiveness of the actions considered by a QMS, which involve both the organization and the activities of the laboratory. Practical examples illustrate the benefits and improvements observed in the laboratory.
Key words: Quality / research / reliability / management / measurement / manpower / methods / materials / machinery / mother-nature
© V. Molinéro-Demilly et al., published by EDP Sciences, 2018

Over recent years, a number of public sector research entities have been adopting a Quality process in order to improve their organization. In France, French standards association (AFNOR) formally recommends adoption of a Quality process by scientists [ 1 , 2 ]. However, implementation of a quality process in a public organization can come up against specific problems not encountered in a private organization [ 3 ]. Research requires both rigour and transparency in the production of knowledge, and involves specificities in terms of objectives, resources and organizational skills that can be very different from those of the industrial sector in which a Quality process has traditionally been found. In view of this, it is clear that the implementation of a Quality Management System (QMS) within a public research organization cannot be carried out in the same way as in industry [ 4 ]. Clearly, the specific challenges that may be encountered in a research laboratory need to be addressed via specific solutions and actions to ensure the success of a QMS.
In the literature, few papers [ 5 – 7 ] deal with the implementation impact of QMS in a research laboratory. Spencer et al. [ 5 ] underline the advantages in Quality assessment of qualitative research for evaluations of research programmes. The quality of scientific research is often uneven and lacking in credibility, making it difficult to make a confident, concrete assertion or prediction regarding evidence for improving practice or consumer outcomes [ 6 , 7 ]. The debate is also due, in part, to the lack of consensus on the specific standards for assessing Quality research. Edmondson et al. [ 8 ] introduce a framework for assessing and promoting methodological fit as an overarching criterion for ensuring quality field research. Baker [ 9 ], Begley et al. [ 10 ], Giesen et al. [ 11 , 12 ], Bareille et al. [ 13 ] show the importance of a Quality process in sciences for improving research management and reliability.
In this paper, we identify the advantages of implementing a QMS in a laboratory of INRA, the French National Institute for Agronomic Research, whose mission is to produce and publish knowledge gained through reliable results, train researchers, offer expertise, create, and innovate.
After presentation of the quality policy of the laboratory, several Quality main actions are developed and discussed using a modified Ishikawa diagram [7Ms: Management, Measurement, Manpower, Methods, Materials, Machinery, Mother-nature (environment)] in order to show the effectiveness of implementing the QMS, which involve both the organization and the activities of the laboratory.
Practical examples are presented to demonstrate the benefits and improvements achieved by implementing a QMS in a research laboratory, as well as the challenges encountered and the solutions proposed to deal with these. The methodology uses the first author's own feedback drawn from three years' experience as Quality Manager in an INRA Laboratory.
2.1 Organization of the laboratory
The research laboratory (or to give it the INRA term, Unit) under observation was created in January, 2012 and is a relatively complex structure, operating under the auspices of three separate Institutions: INRA (French national institute for agronomic research), a School of Engineering (Agrocampus Ouest) specialized in agronomy and horticulture, and a University (University of Angers). As regards INRA, the laboratory is attached to three different scientific divisions, each covering several disciplinary fields where the research constantly explores new ground. The laboratory is the result of the merger of four MRUs (Mixed Research Unit), and currently numbers some 230 staff members organized into 16 teams ( Fig. 1 ). From INRA's point of view, this is a Very large scale unit (VLSU), as the number of staff exceeds 100, whereas the average number of staff in an INRA Unit is 25. However, we have become increasingly accustomed over recent years to Units that merge with a view to pooling resources (i.e. sharing equipment and reducing the number of posts in Research Support Services while giving greater visibility to the Units). The laboratory is therefore of recent formation and has been subjected to extensive structural change.
The laboratory conducts research projects in seeds and horticulture. It is committed to an integrated approach of coordinated effort and expertise in the fields of genetics, epigenetics, genomics, pathology, physiology, ecophysiology, biochemistry, modelling, statistics, and bioinformatics.
Prior to the creation of the laboratory in 2012, the four former MRU (Mixed research unit) teams were located on different geographical sites. Figure 1 also shows the institutional membership of the laboratory staff. The INRA teams had already begun implementation of a Quality process in the year 2000.
MRU 1 had been internally audited by the INRA Quality task force in 2008 in accordance with INRA Guidelines Version 1 [ 14 ]. The result of this audit concerning management responsibility, documentation and resources management was highly complimentary reflecting the considerable efforts the MRU had made to meet the requirements of the INRA Guidelines version 1.
MRU 2, a Biology Resource Centre (BRC) has had ISO 9001 certification [ 15 ] since 2008. This BRC has achieved international renown and has a very dedicated Quality manager.
In MRU 3, a Quality process had been introduced. Quality, equipment and metrology managers were appointed in this research unit.
MRU 4 was operating under the auspices of a University that had not adopted a Quality process for its research departments. The same was true for the teams working for the School of Engineering, which had ISO 9001 certification for academic activities only but not for the research activities. Nevertheless, all university and engineering school teams were using laboratory notebooks, had drawn up operating procedures, conducted equipment inventories, implemented life cycle files or equipment monitoring logs, and observed the minimum requirements concerning external checking of pipettes and weighing scales.
The first one was due to administrative dissimilarities between the three institutions (INRA, the School of engineering and the university). This obstacle has been solved by delegating management of the new VLSU to INRA via a contractual agreement;
The second one concerned the multidisciplinary nature of the scientific community and the need to get individuals with different backgrounds and habits working efficiently together as well as to create synergy around Quality within the laboratory. This necessity had already been identified when the four MRUs were created, and became even more apparent when the VLSU came into being. The laboratory defined an objective of constructing a common QMS for all its research activities. One of the actions decided upon was the recruiting in September 2013 of a Quality manager to work full-time on Quality, health, safety and environment;
The Quality manager's first task was to establish an inventory of the existing situation, before moving the laboratory towards harmonization of all practices, bringing them in line with INRA guidelines version 2 [ 16 ]. However, teams that had made significant progress as regards quality felt that they were being made to regress following the merger and there has been a need to involve and remotivate them via the Quality actions undertaken;
The third one was the geographical spread of the teams. In 2012, all teams were still dispersed over four distant sites. Communication and common working were facilitated when the Institutions that benefit from county council funding received a brand new building, which enabled teams to be relocated to a single site during the summer months of 2015.
2.2 The key to success: a committed Management Board
The success of a QMS depends on the commitment of staff, and most particularly that of top management. This commitment was formally expressed in a Quality policy statement (an obligatory step for any organization with ISO 9001 certification [ 15 ] or EN ISO/IEC 17025 accreditation [ 17 ]). The Quality policy outlines the objectives of the organization and the planned operational rollout of the associated action plan.
Guarantee reliability of measurable results via controlled methods and equipment;
Ensure traceability of research work;
Contribute to long-term conservation of data;
Guarantee quality of biological materials;
Guarantee quality of services provided by Biology Resource Centres (BRC);
Manage samples;
Contribute to human and environmental as well as collaborator risk management;
Ensure appropriate planning and organization of projects;
Harmonize practices, methods and operating procedures common to various teams;
Instigate appropriate and effective improvements.
2.3 Choosing Quality guidelines appropriate to a research organization
Convinced of the absolute necessity of the Quality process in the scientific environment, INRA officially embarked upon the Quality process in the year 2000. The INRA management coordination committee sent out its first Quality policy statement in March of that same year and instigated the INRA Quality task force. In 2005, INRA published its first Guidelines (Version 1) as well as introducing a self-assessment tool for the Units. These first Guidelines comprised five chapters: Quality Management and management responsibility; Documentation; Management of resources; Core activities; and Measurements, Analysis and improvement. In 2006, the first steps towards implementing the Quality process came into effect in INRA support services. A review of actions undertaken between 2000 and 2009 reveals the support given to the Quality process by the INRA Board of Management, the commitment of the research departments (12 out of 14), the commitment of the Units (25% in 2000 rising to 95% in 2004), and the application of international references such as ISO 9001 and EN ISO/IEC 17025 (15) for strategic platforms certified by the National commission for collective Tools (CNOC), as well as ISO 14001 [ 18 ] for Experimental Units, and ISO 9001 [ 15 ] or NF S 96-900 [ 19 ] for certified Biological resource centres.
INRA's next ambition was to extend the Quality process to research activities, thus bringing Quality to the very heart of INRA's activity. In 2012, the INRA Management coordination committee's new 2012–2016 Quality policy emerged. Version 2 [ 16 ] of the INRA Quality guidelines comprises five chapters: Quality management and responsibilities; Conducting research; Management of resources; Control of the documentation; and Measurements, analysis and improvement. This new version of the INRA Guidelines was presented to quality or metrology managers in laboratories.
This new guide is intended to be easy to read, using everyday language to ensure accessibility for the scientific community, since Quality terminology is rather specific and becoming familiar with it can take time. The INRA Quality task force also contributed to the drawing up of the NF X50-553 Standard (management of research activities) [ 2 ] and made sure the INRA Guidelines were consistent with this Standard. The INRA Guidelines deliberately make no reference to customers in order to avoid resistance from the scientific community to a concept commonly associated with the commercialization of knowledge. Version 2 of the INRA Guidelines is about accruement of experience and reinforcing continual improvement. It puts emphasis on conducting research as a process (design, implementation and publication/practical usefulness) with a view to managing and controlling the risks inherent during a research project. At the outset of the project, the person heading the research states the hypotheses involved, defines the experimental protocols, coordinates sampling/analyses/simulations, and interprets data and designates its uses.
The laboratory is required to draw up an inventory of all its research projects and establish research and/or experimental protocols. These protocols cover the objectives defined for the research project as well as the resources necessary to achieve them (methods, materials, resources, installations; persons and entities involved, provisional schedule, critical aspects requiring special attention and procedures for communication, retention period of samples and data, as well as any other specific criteria). The INRA version 2 Guidelines also put emphasis on management of methods: their formalization and validation, and the uncertainties associated with quantitative results. The version 2 INRA Guidelines come with a new dedicated self-assessment tool for the research units and specific tools for the implementation of the Quality process at national level: the INRA Quality task force is coordinated by a network of Quality managers located in centres across 17 different sites in France and the 13 scientific divisions. However, the ideal is not so easy to achieve in reality and many of the scientific divisions that were involved with the first version of the guidelines have since lost interest in the Quality process, and some centres are still without a Quality manager. The effect of this is to isolate the Quality managers in the units, just as these units undergo the process of merging and have growing staff levels.
When it comes to the VLSU, structural complexity complicates smooth coordination, as is evident in the case of the biology laboratory under observation: acceptance of the INRA guidelines needs to be achieved across 16 Laboratory teams (irrespective of the institute individuals belong to), in the centre of INRA Angers-Nantes, and in the three INRA scientific divisions (only one of which has a Quality manager).
At the same time, in the face of such extensive restructuring, the implementation of a QMS could actually be seen as an opportunity, offering the possibility on the one hand of managing risks specific to research activities, and on the other of enhancing cohesion between teams and ensuring that knowledge acquired is put to good purpose.
3.1 Managing the 7 Ms in a laboratory
The research community is agreed on the principle that scientific publications must be founded on reliable scientific data obtained in an environment where all factors capable of influencing the quality of a result (see Fig. 2 ) are tightly controlled [ 20 – 24 ]. These factors can be displayed in the manner of the Ishikawa Fishbone diagram with 7 principal categories (see Fig. 2 ): Machinery, Methods, Materials, Mother-nature (environment), Manpower, Management and Measurement.
Assessing the reliability of research results consists in attributing a confidence level relative to both the obtainment and the use of the results. In the case of research activities, it can be difficult to assess reliability with an appropriate confidence level but the minimum that can be expected is to be in control of all the factors mentioned in Figure 2 . The implementation of a QMS which integrates the principle of the 7 Ms constitutes an opportunity to ensure quality of research results, and to improve and obtain recognition of the work carried out in a research laboratory.
The main actions implemented in the laboratory under observation are described in the following sections, for each of the influence factors illustrated in Figure 2 . All actions that were put into effect came about as a result of the continual improvement dynamic brought to the laboratory by the existence of the QMS.
3.2 Management and Manpower
The QMS constitutes a tool with which to control and steer the activities of the unit.
The laboratory has chosen to adopt an integrated approach to Quality management that includes aspects linked to prevention and sustainable development. A participative management style was chosen by the Management Board for implementation of the QMS [ 23 ] with the intention of encouraging inter-team and inter-discipline exchange. In September 2013, the Quality manager was appointed with a brief to implement and steer a Quality system common to all laboratory research teams. He has extensive independent powers to enable him to fulfil this brief, as well as an operating budget. He attends monthly steering committee meetings for the laboratory, at which any matters relating to Quality and prevention can be raised if necessary.
The danger was of the Quality manager finding himself shouldering this huge task single-handed. With the support of the laboratory manager, a Quality network was created with more than 60 researchers of the laboratory: the laboratory manager, the 16 research team leaders, the 16 Quality representatives (one per team), and 35 Equipment and Metrology representatives. The Quality representatives meet every two months. A mission letter was sent to the Quality manager, the Quality representatives and the Equipment and metrology representatives.
In order to help the laboratory's Quality manager and Quality representatives to deploy the Quality process among research teams, the Quality manager made good use of the commitment of students on work experience in the laboratory. The advice of their mentor, a specialist in Quality management and metrology, went a long way in ensuring implementation of the QMS was possible with the cooperation of all concerned. This tight collaboration had a number of positive offshoots and several actions have been dealt with, such as process mapping (see Fig. 3 ), a Quality manual, and procedures for document and equipment control, all of which advances formalization of process and operating procedures [ 15 ].
To ensure reliability of research results, it is essential from the outset to pay due regard to Human Resource management [ 23 , 25 ]. This consists in identifying the functions and skills required (in terms of knowledge, know-how and experience) and hence training needs, welcoming new recruits and retaining records of initial and ongoing training.
Every two years, at the activity meetings held between the members of staff managed by INRA and their line managers, a review is made of the different activities, of prospects, of skills acquired and needing to be developed, and of training needs. A training programme is thus established for the laboratory, and priorities are set in line with the laboratory's Guidelines. It has been noted that staff training in Quality and metrology needs to be developed [ 25 , 26 ] as the lack of this is slowing down the progress of the laboratory.
3.3 Methods
When analysing test results, researchers need to have at their disposal all the information that could have an influence on results [ 20 ]. Therefore the formalization of methods is essential. This consists in noting down all sample collection, measurements, analysis of apparatus used, kit lot numbers, the samples themselves, their identification numbers, storage temperatures, etc. In accordance with INRA Guidelines, these operations are written down in a laboratory notebook when the method is being set up; the operating procedure is in place once the method has been fully defined and is workable. INRA is in the process of developing electronic notebooks to further encourage their use by scientists and facilitate the traceability of information. The use of laboratory notebooks by scientists in INRA laboratories is a long-standing practice. Once a method is deemed reliable, it is transcribed in the operating procedure (using the model defined by the laboratory).
In the laboratory, research teams formalize the validation steps of their methods in accordance with the instructions in INRA guidelines version 2. In other words, the evidence is created to confirm that the method utilized is appropriate to the question being treated; any question of the conditions required to produce interpretable results with a known level of uncertainty can be answered.
Data management is also a crucial matter, one which the bioinformatics team at the laboratory would like to improve. The development of a Laboratory information management system (LIMS) is underway and will improve the management of samples (identification, localisation) tested and the traceability of their associated data. The objective is to be able to find easily where a sample comes from, whose it is, to which methods it relates, everything that has been done throughout its life cycle and how to use dispose of it [ 16 , 17 ].
The LIMS will also be used for the management of equipment (which will facilitate the work of the Equipment and Metrology Representatives), and also consumables so as to avoid the use of different product or reagent lots where this would impact upon results.
Document management is another essential factor that has to be properly handled by the laboratory. The laboratory lists the operating procedures that need to be formalized, schedules their realization, has them written up, and disseminates them via any means considered appropriate to enable them to be used in operational conditions. The laboratory defines and utilizes template documents for the writing of operating procedures. An initial list of documents has been created. It is updated by the Quality representatives in such a way that every scientist can be aware of all operating procedures in existence as well as of modifications to them. Documents created and validated as part of the QMS are made available for use by means of a document management tool. This tool is encountering a certain amount of resistance as some scientists object to this general availability of what they consider to be their own documents.
All researchers know that it is essential to describe precisely their methods and to validate and to improve their scientific works. It is also important to record correctly the validation methods used and the associated results and data. For the continuous improvement of the research laboratory, the useful QMS tools allow the laboratory to also share knowledge and better capitalize on a know-how.
3.4 Machinery and Measurement
The laboratory has responsibility for managing equipment that is subject to regulations or is identified as having an impact on the quality of research results. This empowers it to ensure that the purchasing, maintenance, calibration, and verification of equipment are conducted appropriately [ 27 – 29 ].
When it was created in 2012, the laboratory had eight different types of inventory for the listing of equipment. Critical equipment was not always identified as such and several different service-providers could be involved in the regulatory control of a single apparatus type depending on which teams used it. It was a matter of high priority to standardize the inventory and equipment management systems (pertaining to information such as model, make, serial number, commissioning date, person responsible, etc.). It took almost two years to develop an internal network with a referent for each team (a matter of 35 Equipment and metrology representatives) and collectively define their brief: to ensure regulatory verifications with a view to prevention (autoclaves, fume hoods, centrifuges, oxygen meters, etc.) and/or metrological verification and calibration (weighing-scales, pipettes, thermometers, incubators, water baths, etc.).
Each critical device identified has its own service-life file enabling the tracing of incidents and the monitoring of maintenance, verification, and/or calibration. When a piece of equipment fails a conformity check, the validity of all preceding results must be re-established. All operations pertaining to equipment are covered in the common equipment management and control procedures, and in equipment user, maintenance, calibration, verification and monitoring instructions. An annual schedule for both internal and external verification of critical equipment has been set up [ 27 ]. For example: weighing-scales identified as critical are periodically checked in-house with calibration weights and control charts [ 28 – 33 ]. The weighing-scales are also verified annually by an external service-provider. Weighing-scales that are identified as non-critical undergo in-house verification only. In molecular biology, pipetting of reagents is a critical activity which can have a significant impact on a result, especially where small volumes are concerned. Due to the number of pipettes in use, these make up a significant proportion of the equipment to be checked. A joint decision has therefore been made to perform verification in-house for pipettes with a volume above 10 μL and to use an external service provider for pipettes with a volume below 10 μL as well as for multichannel pipettes [ 33 , 34 ]. For temperature, the laboratory has acquired a reference thermometer, calibrated annually, with which to verify operational laboratory thermometers. For verification of more complex equipment such as thermal cyclers, a workgroup has been set up with the aim of developing a procedure to be used for in-house verification.
For machines that carry a degree of safety risk to the user, such as centrifuges, autoclaves, etc., regulatory checks are compulsory at the intervals defined in the relevant regulations. For autoclaves, an authorization given by an external body is required.
3.5 Mother-nature and Materials
The INRA guidelines require units to ensure proper monitoring, recording, and if possible control of ambient conditions when these have an impact on the quality of research results.
Discussions are currently underway with Equipment Managers in charge of freezers and cold rooms on the subject of identifying critical aspects requiring special attention where samples need to be stored at −80 °C. The laboratory stores pathogenic agents (bacteria and fungi), seeds, leaves, twig fragments, pieces of fruit, and also DNA, RNA, and proteins. In order to control the risks associated with poor cold storage conditions (at temperatures of −80 °C, −20 °C and +4 °C), several requirements have been pinpointed: the requirement for an on-site power generator, the installation of −80 °C freezers in an air-conditioned room, of a monitoring system for each freezer and cool room to ensure reliability (for a backup −80 °C freezer, for maintenance of freezers and cool rooms by an external company with a rapid response time in the event of failure) and, finally, for an in-house team capable of dealing with failures at weekends.
The INRA version 2 guidelines require laboratories to ensure correct cold storage of samples (cryopreservation, −80 °C, −20 °C and 4 °C). To satisfy this requirement the laboratory is in the course of defining a clear policy concerning management of freezers and refrigerators, as well as standardized numbering for all samples within the laboratory in order to ensure their traceability. The Quality representatives are also discussing protocols for the collection and acquisition of samples, types of packaging (e.g. tubes, plates, bottle, boxes, etc.), and methods of identifying the samples. A disposal policy for samples (post publication, at end of project.) and the scheduling of cleaning days are also under discussion.
The laboratory is responsible for the traceability of consumable and other products (chemical and phytosanitary products, solvents, biological reagents, etc.). The question of traceability is not handled in exactly the same way by every team. Nevertheless, all teams adhere to use-by dates and required storage conditions. The storage of consumables, other products and reagents must conform to regulations and manufacturer specifications. After the merging of the research units, which saw more than half the research teams move to a new building and the construction of new greenhouses, a massive sorting of chemical products was undertaken, with comprehensive inventories being drawn up and appropriate storage made available: clearly defined product bins ensure that acids, bases, inflammables and toxic and carcinogenic, mutagenic, toxic to reproduction (CMR) substances are kept separately from each other. Ventilated cabinets have been purchased for all the laboratory buildings. A special room dedicated to the preparation of phytosanitary products has been built near the new greenhouses. Chemical safety information has been centralized in a computerized folder to which everyone has access.
4.1 Measuring effectiveness of the system
The effectiveness of the system is measured via internal audits and the annual self-assessment tool implemented by the INRA Quality Task Force. An internal audit is organized by the INRA Quality task force every five years, a year before the HCERES (French High Council for Evaluation of Research and Higher Education) assessment of the laboratory. To the overall laboratory assessment are adjoined the Quality audit report, the ensuing action plan, the results of the action plan and the quality indicators selected. Nevertheless, it would be a positive step if the bodies assessing the laboratory were to pay closer attention to the efforts made by the laboratory towards enhancing reliability of results. In order to foster a more self-critical view and further the objectives of continual improvement, it is intended that the laboratory will, for the first time, conduct a Quality review at the end of the year to evaluate the Quality actions undertaken, assess their effectiveness, and define new objectives for the coming year based on the indicators defined by the laboratory for each of its processes. It is hoped by this means to give individuals a real opportunity to enhance their relationship with the Quality system and to instil dynamism in the pursuance of improvement. The Quality process is progressing well and awareness of the benefits attached to a QMS is growing within the laboratory.
4.2 Effect of QMS on organization of the laboratory
The INRA Management coordination committee recommends laboratories to undergo a Quality audit a year ahead of the HCERES assessment which takes place every five years. In response to the wish of management, therefore, an INRA internal audit was held in the VLSU in March, 2015 organized by the INRA Quality task force. The auditors took the time to audit every team (on every site) in accordance with the different requirements of the INRA version 2 guidelines. This very pedagogical action allowed scientists to measure in real terms the improvements made or needed to be made by their teams. This internal audit made it possible to draw up individual team-oriented action plans based on specific needs, followed-up with an action plan for the laboratory as a whole. The actions decided upon were prioritized according to three objectives: improvement of documentation management, of equipment management, and of cold-stored samples management (cryopreservation, −80 °C, −20 °C, 4 °C and lyophilisation). These objectives were then confirmed in the management mission statement, which was updated in 2016. The audit was therefore a very effective means of continuing to involve teams in the Quality process and of facilitating interaction between the teams and the Quality manager, and was also a means through which the collective objectives of the laboratory could be developed. This is in keeping with the concept of participative management put into effect by the laboratory management board.
4.3 Effect upon commitment and motivation of laboratory staff
The fact that the laboratory is under no obligation to pursue the certification objective means the scientific community may suffer a lack of motivation. However, this is actually a very positive situation: it allows staff the time it takes to become fully conversant with the new managerial process, one which actively encourages the participation of individuals, promotes a shared outlook, and fosters an ongoing critical regard of the organization of the laboratory. The process management constitutes a tool with which to steer laboratory activities with regard to key performance indicators. It involves every member of laboratory staff, favouring continual improvement of the operation, organization, and practices of the research laboratory via the Quality policy, Quality objectives, and results of self-assessment and audits.
In order to deepen the commitment of its scientists to the Quality process the laboratory is developing, in conjunction with its closest partners, a network of Quality managers, which it is intended will be broadened in order to benefit from the experience of other Organizations, such as INSERM (French National Institute of Health and Medical Research) and CIRAD (French Agricultural Research Centre for International Development). As the Quality process is not inscribed in the official duties of staff, implementation is not easy. Fortunately, the laboratory is able to count upon the commitment of its willing staff.
Recognition for individuals who participate in collective tasks needs to be increased. While the contribution of individuals to collective tasks such as prevention and risk management does come up at activity meetings and in competition for promotion, staff generally feel that only their scientific contribution (in the form of scientific communication and publication) is taken seriously. Only this, it seems, has any real effect on career development. In the light of this it is easy to understand why a number of laboratory staff takes little or no part in this type of collective activity.
This paper presents the different actions involved in setting up a QMS in a very large French research laboratory (very large scale unit) through a voluntary approach.
This paper clearly illustrates the effectiveness of the actions considered by looking at the 7M method and giving practical examples which involve both the organization and the activities of the laboratory.
Many improvements were made at the time of setting up the QMS in the laboratory. These have had a positive impact on the functioning and the activities of the laboratory.
Putting a QMS into place certainly improves the functioning of the laboratory since it provides information on where people are, what they are doing, how they are doing it, how what they do is being checked and how things can be anticipated. Quality tools allow laboratory staff to be accompanied in a spirit of continual improvement in order to maintain effectiveness and robust activities of research of the laboratory.
The management of quality also aims at opening up discussion so researchers can put meaning into their work and improve their research activities. The participative management aspect of the Quality process encourages a shift, initially on an individual basis but consequently at organization level, from wanting change to enjoying it. This participative style of management brings together different perspectives that enable anticipation, cooperation and innovation.
The QMS is still young and more needs to be achieved for it to be completely operational and cover all the processes linked to the activities of the laboratory. All the laboratory staff needs to acknowledge the QMS and become involved for it to function correctly. Efforts to increase researchers' awareness are continuing in the laboratory and in field work by showing, step by step, that the QMS exists to enable the laboratory and its quality staff to continue to progress from an organisational as well as scientific point of view.
Although it enjoys the support of the laboratory management, the implementation and development of a QMS is encountering resistance both from scientists and from the Institutions, notably in the latter case, for financial reasons: the IT tools, for example, that improve the management of documentation, equipment, consumables, and chemical products take time to develop satisfactorily and necessitate a training budget. And yet these tools help underpin the management of collective intelligence. Currently, the financial support of the Institutions contributes to the cost of fluids and research projects but provides nothing for the development of structural tools. Despite the economic pressures, scientists within the laboratory do willingly support the QMS. The laboratory could also take its work on the validation of the methods further, increasing emphasis on the estimation of uncertainties associated with results. Among other aspects that need to be improved are the control of outsourced activities and the evaluation of supplies and suppliers. It is perhaps useful at this point to refer to the experience of other laboratories: despite the difficulties encountered during the implementation phase of a QMS, of all those questioned who had been in a position to observe the changes to the organization of their laboratories, none expressed a wish to backtrack. This seems to reinforce the claim that a QMS, while admittedly demanding a certain effort from everybody in the laboratory during the implementation phase, does serve to enhance reliability and improve the functioning of a laboratory.
- A. Jaime, M. Gardoni, D. Vinck, Quality Management, Framework of Knowledge Capitalization at Research Organizations IAMOT 2004 (International Association for Management of Technology, USA, 2004) [Google Scholar]
- NF X50-553, Management des activités de recherché (AFNOR, Paris, 2014) [Google Scholar]
- A. Matei, C. Antoni, The new public management within the complexity model, Procedia Soc. Behav. Sci. 109 , 1125–1129 (2014) [Google Scholar]
- G. Gruening, Origin and theoretical basis of new public management, Int. Public Manag. J. 4 , 1–25 (2001) [Google Scholar]
- L. Spencer, J. Ritchie, J. Lewis, L. Dillon, Quality in Qualitative Evaluation: A Framework for Assessing Research Evidence (National Centre for Social Research, London, 2003) [Google Scholar]
- What Are the Standards for Quality Research? Technical Brief Number 9, National Center for the Dissemination of Disability Research, 2005 [Google Scholar]
- R.J. Shavelson, L. Towne, Quality Research in Education (National Research Council, National Academy Press, Washington, 2002) [Google Scholar]
- A.C. Edmondson, S.E. Mcmanus, Methodological fit in management field research, Acad. Manage. Rev. 32 , 1246–1264 (2007) [Google Scholar]
- M. Baker, Quality time. It may not be sexy, but the quality process is becoming a crucial part of lab life, Nature. 529 , 456–458 (2016) [CrossRef] [PubMed] [Google Scholar]
- C.G. Begley, A.M. Buchan, U. Dirnagl, Robust research: Institutions must do their part for reproducibility, Nature 525 , 25–27 (2015) [CrossRef] [PubMed] [Google Scholar]
- E. Giesen, Quality management for robust and reliable research, Int. J. Metrol. Qual. Eng. 6 , 407 (2015) [CrossRef] [EDP Sciences] [Google Scholar]
- E. Giesen, Ethical and efficient research management: a new challenge for an old problem, Int. J. Metrol. Qual. Eng. 6 , 406 (2015) [CrossRef] [EDP Sciences] [Google Scholar]
- R. Bareille, B. Baudouin-Massot, M.P. Carreno, S. Fournier, N. Lebret, I. Remy-Jouet, E. Giesen, Preventive actions to avoid questionable research practices. Use of EERM (Ethical and Efficient Research Management) during arrival and departure of a co-worker, Int. J. Metrol. Qual. Eng. 8 , 10 (2017) [Google Scholar]
- INRA Quality Mission, INRA Quality Guidelines, Version 1. 2003-2005. INRA, contact: qualite[at]inra.fr. [Google Scholar]
- ISO 9001, Quality Management Systems − Requirements (2015) [Google Scholar]
- INRA Quality Mission, INRA Quality Guidelines for Research and Experimental Units (2013), Version 2. INRA Sciences and impact, contact: qualite[at]inra.fr. [Google Scholar]
- EN ISO/IEC 17025, General Requirements for the Competence of Testing and Calibration Laboratories (2005) [Google Scholar]
- ISO 14001:2015, Environmental Management Systems − Requirements with Guidance for Use (2015) [Google Scholar]
- NF S96-900, Qualité des centres de ressources biologiques (CRB)-Système de management d'un CRB et qualité des ressources biologiques (AFNOR, Paris, 2011) [Google Scholar]
- S. Belouafa, F. Habti, S. Benhar, B. Belafkih, S. Tayane, S. Hamdouch, A. Bennamara, A. Abourriche, Statistical tools and approaches to validate analytical methods: methodology and practical examples, Int. J. Metrol. Qual. Eng. 8 , 9 (2017) [CrossRef] [EDP Sciences] [Google Scholar]
- L. Martin, Ch. Baudassé, N. Leménager, M.-F. Thévenon, The metrological approach: a major key factor for the accreditation and continuous improvement of the wood preservation laboratory of Cirad, Int. J. Metrol. Qual. Eng. 4 , 117–120 (2013) [CrossRef] [EDP Sciences] [Google Scholar]
- F.M. Beyer, Language and strategies at work studied through the implementation of quality procedure standards, Sociol. Trav. 41 , 235–54 (1999) [Google Scholar]
- ISO 10018, Quality Management–Guidelines on People Involvement and Competence (2012) [Google Scholar]
- ISO 10012, Measurement Management Systems–Requirements for Measurement Processes and Measuring Equipment (2003) [Google Scholar]
- Guide on equipment qualification: http://www.edqm.eu/en/quality-management-guidelines-86.html . 2015 [Google Scholar]
- L. Martin, M.-F. Thévenon, Evaporation as an ageing procedure prior to wood preservative biological testing: when standardization needs metrology, Int. J. Metrol. Qual. Eng. 5 , 405 (2014) [CrossRef] [EDP Sciences] [Google Scholar]
- G. Gomah, A traceable time interval measurement with a reduced uncertainty, Int. J. Metrol. Qual. Eng. 6 , 301 (2015) [CrossRef] [EDP Sciences] [Google Scholar]
- S.B. Amara, J. Dhahri, N.B. Fredj, Control chart limits based on true process capability with consideration of measurement system error, Int. J. Metrol. Qual. Eng. 7 , 401 (2016) [CrossRef] [EDP Sciences] [Google Scholar]
- S.F. Beckert, W.S. Paim, Critical analysis of the acceptance criteria used in measurement systems evaluation, Int. J. Metrol. Qual. Eng. 8 , 23 (2017) [CrossRef] [EDP Sciences] [Google Scholar]
- J.-M. Pou, L. Leblond, Control of customer and supplier risks by the guardband method, Int. J. Metrol. Qual. Eng. 6 , 205 (2015) [CrossRef] [EDP Sciences] [Google Scholar]
- M. Wiederhold, J. Greipel, R. Ottone, R. Schmitt, Clustering of similar processes for the application of statistical process control in small batch and job production, Int. J. Metrol. Qual. Eng. 7 , 404 (2016) [CrossRef] [EDP Sciences] [Google Scholar]
- ISO 8655-1, Piston-operated Volumetric Apparatus-Part 1: Terminology, General Requirements and User Recommendations (2002) [Google Scholar]
- ISO 8655-2, Piston-Operated Volumetric Apparatus-Part 2: Piston Pipettes (2002) [Google Scholar]
- ISO Guide 30, Reference Materials-Selected Terms and Definitions (2015) [Google Scholar]
Cite this article as : Valérie Molinéro-Demilly, Abdérafi Charki, Christine Jeoffrion, Barbara Lyonnet, Steve O'Brien, Luc Martin, An overview of Quality Management System implementation in a research laboratory, Int. J. Metrol. Qual. Eng. 9 , 2 (2018)
All Figures
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.
- Open access
- Published: 28 October 2024
The global research landscape and future trends in healthcare Total Quality Management
- Zhiyuan Hu 1 na1 ,
- Richard Szewei Wang 2 na1 ,
- Xiaoping Qin 1 ,
- Yu-Ni Huang 3 ,
- Herng-Chia Chiu 4 , 5 ,
- Yuanli Liu 1 na2 &
- Bing-Long Wang 1 na2
Archives of Public Health volume 82 , Article number: 193 ( 2024 ) Cite this article
278 Accesses
Metrics details
Total Quality Management (TQM) is instrumental in augmenting the quality and efficacy of healthcare service delivery, but a comprehensive evaluation of present and evolving TQM research trends within healthcare research articles is notably absent. This study provides an insightful view into the prevailing international scenarios and upcoming research frontiers in healthcare TQM research field, utilizing bibliometric mapping through VOSviewer. Drawing data from 360 publications in the Web of Science core citation database, it delineates a steady growth in the field over the last 30 years. Research outputs span 51 countries and regions, with notable contributions from the United States, United Kingdom, Netherlands, and Italy. The top five research institutions and numerous authors predominantly hail from the United States. Key keywords in near years encompass healthcare safety, healthcare quality assurance, quality indicators, and the application of Six Sigma management principles. This exploration serves as a pivotal reference for understanding the global research landscape and future trends in healthcare TQM, particularly in guaranteeing quality and safety. Future scientific endeavors will build upon these focus areas, exploring and connecting research gaps in more specialized fields.
Peer Review reports
Introduction
Total quality management in healthcare and research focus.
Total Quality Management (TQM) is one of the most prominent developments in management for the over past two decades [ 1 ]. From the 1990s onwards, TQM saw widespread application in management practices in developed nations and regions [ 2 , 3 ].
TQM is recognized as a perpetual process where a quality-focused management style is adopted [ 4 ], is an integrated process involving all systems and employees in a continuous effort to improve quality, reduce cost, and enhance service to the customer [ 5 ]. Every organization member is tasked with establishing and adhering to standards in their roles to not only meet but often surpass the anticipations of both internal and external customers, thereby facilitating enduring organizational prosperity [ 6 ]. While initially formulated for industrial and manufacturing entities, numerous researches suggests that TQM’s application has broadened to encompass sectors serving societal and population needs, notably, including healthcare service and education industries [ 7 , 8 , 9 , 10 ].
TQM in healthcare management is a comprehensively-structured and measurable approach to organizational management that seeks to offer a high-quality healthcare service, which is the aim of the quality management programs in all hospitals [ 1 , 11 ]. TQM involves the application of qualitative and quantitative methods to improve the effectiveness, efficiency, and flexibility of all processes within a healthcare organization [ 12 ].It emphasizes the importance of a patient-centric approach, where patient satisfaction is a primary focus [ 13 ]. TQM includes a culture of continuous improvement, where co-operation, communication, and employee involvement are crucial [ 14 , 15 ]. By integrating TQM principles, healthcare services aim to enhance patient care, reduce medication errors, and ensure a high level of staff satisfaction and engagement [ 16 , 17 ]. Ultimately, the goal of TQM in healthcare is to increase the quality of care, while also controlling costs, leading to overall organizational excellence in the healthcare sector.
An exhaustive exploration into the global research trajectory pertaining to TQM in healthcare service organizations is a gap and is necessary [ 13 ]. The primary objective of this research is using bibliometric methods to explore the development of academic publications on TQM in the healthcare services, a notably complex sector and delivery of service is the fragmented care [ 18 ]. The research questions focus on the following: What are the salient themes and hot topics in TQM in healthcare research field? What are the current situations, gaps and future directions in this research field?
Literature review on healthcare Total Quality Management bibliometric study
In 2017, Voon-Hsien Lee and Jun-Jie Hew published an article titled “Is TQM Fading Away? A Bibliometric Analysis of a Decade (2006–2015)” in the International Journal of Services, Economics and Management. They conducted a retrospective bibliometric analysis of TQM academic research from 2006 to 2015. Their findings indicated that, within the specified research timeframe, many journals, countries, and research institutions were still enhancing the state of TQM knowledge, and the citations of recently published TQM articles were increasing. They also encouraged researchers to integrate TQM with specific application disciplines for further study [ 7 ].
In 2018, Alzoubi MM, Hayati KS, and others published an article titled “Total quality management in the health-care context: integrating the literature and directing future research” in the journal “Risk Management and Healthcare Policy.” They conducted a systematic review of the literature, meticulously reviewing 25 articles focused on TQM in the healthcare context, and concluded that there is “a dearth of studies” in this area [ 13 ].
In 2020, an article titled “A Bibliometric View on the Use of Total Quality Management in Services” was published in Total Quality Management & Business Excellence by Chen Zhang, M. R. Moreira, et al. They employed bibliometric methods to examine the landscape of TQM research in the service sector and posited that TQM is more widely applied in the healthcare service compared to other industries [ 19 ].
Existing bibliometric and review studies have primarily focused on the researches of TQM in the service and management, with research papers typically appearing in management journals. To date, there has not been a dedicated bibliometric analysis specifically addressing TQM research in the healthcare service, published in healthcare research journals. Consequently, this study represents an innovative contribution to the field.
Materials and methods
Data source.
Web of Science (WoS) core collection was chosen as the foundational database for analysis. WoS is a platform owned by Clarivate Analytics [ 20 ], and is the largest and most comprehensive core journal citation index data service platform, covering a wide range of disciplines and studies [ 21 , 22 ]. In this study, informed consent was not mandated for data collection, given the utilization of secondary data, devoid of any personal information.
Search strategy
A Boolean syntax search strategy was employed in the Web of Science core collection database using TS=((TQM OR Total-Quality-Management OR TQC OR Total-Quality-Control)AND (healthcare OR hospital OR medical OR medicine)), resulting in the retrieval of 360 articles, on the date of 9th of September, 2023. Ten articles published in 2023 and two articles before the year 1990 were excluded, because the two articles published before 1991 were outliers (one from 1970 and one from 1974). This reduction resulted in a total of 348 articles being considered for further analysis. Of them we excluded 22 documents that did not fall into the categories of ‘article’, ‘review article’, ‘proceeding paper’, or ‘early access’. Finally, 326 articles were considered for the bibliometric analysis of this study, according to PRISMA principle (Fig. 1 ). The details of the articles downloaded from the WoS database include title, author(s), year of publication, country or region, affiliation, journal, research orientations and keywords.
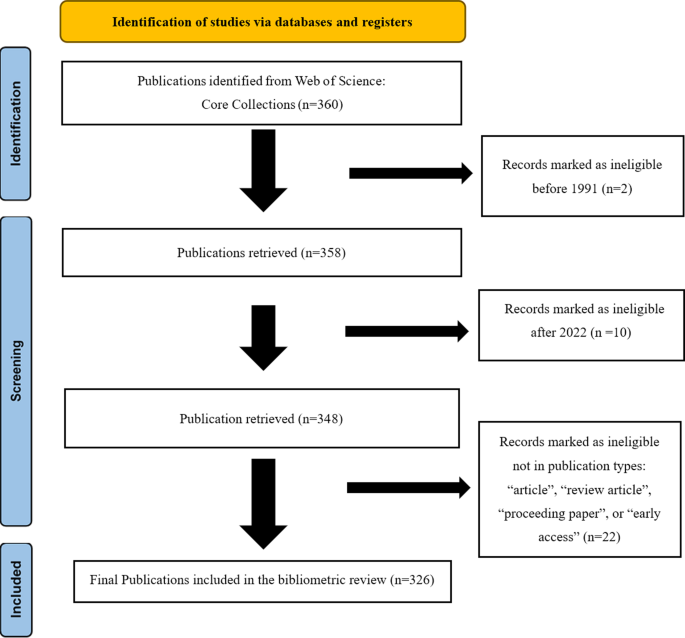
The PRISMA flowchart of the inclusion and exclusion of the database. (1991–2022)
Statistical methods
Our research employed statistical methods, including descriptive and bibliometric analysis. Descriptive statistical methods were used for analyzing and summarizing raw Web of Science (WOS) data, including the analysis of publications by year, curve fitting for the description of global publication trends over time. Descriptive statistics and plotting of publications distributed across different countries and regions, as well as the publication quality in this research field from a bibliometric perspective for different countries, including citation counts, average citation counts, and the H-index as a reflection of quality, and also identified the ranking of publication numbers for different journals, WOS research fields, key authors, and significant publishing institutions [ 23 ].
Bibliometrics is an analytical tool that involves specific measurements aimed at quantifying scientific and technological production through published articles [ 24 ]. Bibliometric methods introduce quantitative rigor into the subjective analysis of literature. By employing quantitative techniques to describe, evaluate, and monitor publications, they enable clear, systematic, and replicable review processes that enhance the quality of reviews and map research fields, thereby avoiding the subjective biases inherent in manual reviews [ 25 ].
There are two main uses of bibliometric methods: performance analysis, which aims to assess the research and publication performance of individuals and institutions; and scientific mapping, which uses visualization tools to reveal the structure and evolution of scientific fields [ 25 , 26 ].
In this study, VOSviewer is employed due to its user-friendly nature and stability while running, which aligns well with the needs of preliminary research where visualization methods can quickly provide an overview of a scientific research field and enable the exploration of numerous data relationships [ 27 ]. As open access software, VOSviewer is also freely available to researchers with limited resources [ 28 ]. Developed by Van Eck and Waltman, VOSviewer is designed to construct and visualize bibliometric networks [ 29 , 30 ]. This software supports the analysis of various factors within these networks, such as journals, researchers, or individual publications, and allows the creation of mapping networks using different relationships, including citations, co-citations, co-authorship, or co-occurrences [ 29 ]. VOSviewer simplifies the visualization and exploration of these metric networks for research purposes [ 30 , 31 ]. Keywords are essential in research articles, containing the most core information [ 31 ]. Systematic analysis of keywords in a particular research area helps to clearly understand the trends and hotspots in that field [ 32 ]. Additionally, co-occurrence analysis, which assesses the relationship between identified keywords based on the number of publications, is an effective method for exploring future trends and hotspots in research areas of interest.
Global publication trends
After applying the search criteria, we retrieved a total of 326 articles. The number of publications has increased since 1991, with 2 publications, and reached 24 publications in 2018 (Fig. 2 a). It can be referred from the graph that there was a period of publication stagnation between 1993 and 2014.
According to Fig. 2 b, it can be observed that the curve does not fit the data well; when using a logarithmic curve fit, resulting y = 7-16exp (0.0185x) to describe the relation between publication number by year, the R-squared value is only 0.1003.
Mainly, the development of this research field is characterized by a wave-like progression. This research field progressed since 1990, rising from 2 to 8–14 publications annually, maintaining a high level from 1993 to 2002. A downturn occurred from 2003 to 2005, with fewer than 8 publications annually. From 2006 to 2018, a second wave of rapid growth emerged, peaking at around 10 publications annually from 2011 to 2019 (excluding the outlier of 4 in 2016), with 2018 witnessing a high of 24 publications. Despite a low of 5 publications in 2020, the significant increase to 16 papers in 2022 indicates sustained interest in TQM research within healthcare management.

( a ) The number of publications on healthcare TQM by years; ( b ) The annual publication volume and trend prediction on healthcare TQM; ( c ) Distribution map of the top 10 countries or regions with the most publications on healthcare TQM; ( d ) The top 10 countries or regions with the highest publication number on healthcare TQM. (1991–2022)
A total of 51 countries and regions have published publications in the field of research. The top contributors in this field are shown in Fig. 2 b and c. The United States has made the most significant contribution with 131 publications; followed by Germany with 42 publications; the United Kingdom with 20 publications; and Netherlands and Canada with 19 publications, respectively. (Figure 2 c and d)
The USA leads significantly in publication output in this field, followed by other major publishing countries, which are primarily European nations and China.
The United States has the highest total number of citations (5,134) among all included publications, while England ranks second (967), followed by the Netherlands (933), Italy (828), and Australia (584) (Fig. 3 a).Publications from Italy have the highest average number of citations (55.2), followed by the Netherlands (49.11), Australia (48.67), England (48.35), and the US (39.19) (Fig. 3 b).The US outranks other countries and regions with the highest H-index of 33, followed by the Netherlands ( n = 11), England ( n = 11), Italy ( n = 10), and Germany ( n = 10) (Fig. 3 c).
The USA leads significantly in total citation counts and H-index rankings, ahead of other countries. The H-index indicates both a large scale and strong quality. However, in terms of average citations per paper, Italy, the Netherlands, and Australia rank in the top three, reflecting a higher quality in terms of citations.

( a ) Top 10 countries or regions with the highest total number of citations on healthcare TQM; ( b ) Top 10 countries or regions with the highest average number of citations per publication on healthcare TQM; ( c ) Top 10 countries or regions with the highest H index on healthcare TQM. (1991–2022)
Analysis of publications
Figure 4 a shows the top ten journals with the highest number of publications.
The Journal of Applied Clinical Medical Physics published the most articles/reviews (14 publications), surpassing other journals in this field. The second highest number of publications came from Clinical Chemistry and Laboratory Medicine, Accreditation and Quality Assurance, Journal of Continuing Education in The Health Professions, with a total of 10 articles/reviews. Additionally, there were 9 publications in Journal of Operations Management, 7 in Biochemia Medica, and 7 in Military Medicine.

( a ) The top 10 journals in terms of publication quantity on healthcare TQM; ( b ) The top 10 research topics and orientations on healthcare TQM; ( c ) The top 10 authors in terms of publication quantity on healthcare TQM; ( d ) The top 10 institutions in terms of publication quantity on healthcare TQM. (1991–2022)
Figure 4 b shows the top 10 research topics related to TQM in healthcare services. The most common areas of research were Health Care Sciences Services (69 publications), General Internal Medicine (41 publications), Public Environmental Occupational Health (29 publications), Medical Laboratory Technology (27 publications), and Radiology Nuclear Medicine Medical Imaging (25 publications).
This reflects research papers focusing primarily TQM in services management, followed by studies in fields such as internal medicine, occupational health, and medical experimental technologies. It highlights that management topics constitute the predominant focus of TQM research.
Figure 4 c shows the top ten authors with the most publications in this field. Among them, Libeer JC ranked first with four articles/reviews, and Bissonnette JP, Cohen DJ, Ehrmeyer SS, Freniere N, Goldschmidt HMJ, Laessig RH, Lippi G, Pomer-Leau-Dalcourt N, Villarreal-Barajas JE, Vogt W followed with three published articles/reviews.
The University of California System, and the University of Wisconsin System have the highest number of publications, with 11 publications each. The US Department of Veterans Affairs has ten publications, followed by VHA (Veterans Health Administration) with nine publications. The top 10 institutions with the highest number of publications are presented in Fig. 4 d. This reflects that university research still has the highest publication volume in this area, followed by various other types of research institutions.
Co-authorship mapping analysis
Co-authorship analysis demonstrates the correlation between items based on the number of co-authored documents [ 33 ], which is a potent instrument for evaluating collaboration patterns and identifying leading scientists, countries, and organizations [ 34 ].
In Fig. 5 a, VOSviewer was used to analyze 61 authors with at least two publications. The top 11 authors with the highest TLS scores are shown in the red cluster on the right side of the figure, represented by Freniere, Normand (citation count = 19), Bissonnette, Jean-Pierre (citation count = 18), and others. VOSviewer also shows three authors with the highest citations (citation count = 479): Friedman, Susan M., Kates, Stephen L., Mendelson, and Daniel A. (all three TLS = 4). They are in the light blue cluster on the upper part of the image.
According to the Fig. 5 a, we can observe that the red group forms the largest collaborative authorship cluster, they come from various research institutions and medical centers across Canada, forming the most prominent research group with evident connectivity strength in this field.

( a ) Co-authorship analysis on healthcare TQM (n = 61); ( b ) Co-authorship analysis of countries and regions on healthcare TQM (n = 25); ( c ) Co-authorship analysis of institutions on healthcare TQM (n = 79). (1991-2022)
A total of 25 countries and regions with a minimum limitation of at least three publications were identified and analyzed using VOSviewer (in Fig. 5 b). The top five countries and regions with the highest TLS scores are presented as follows: the Netherlands (TLS = 30 times), the United States (TLS = 30 times), Germany (TLS = 29 times), Sweden (TLS = 22 times) and Italy (TLS = 19 times).
This finding aligns with the analysis of publications where the USA dominates in terms of the highest publication volume. Institutions and authors from the USA are the most frequent collaborators in multinational research articles.
As shown in Fig. 5 c, a total of 79 institutions had at least two publications included in the study and were analyzed using VOSviewer. In addition, University of Calgary (TLS = 30 times), Tom Baker Cancer Clinic (TLS = 29 times), University of Toronto (TLS = 29 times), Juravinski Hospital and Cancer Centre (TLS = 28 times) and Princess Margaret Cancer Centre (TLS = 28 times) were the top five institutions with the highest TLS scores.
These highly collaborative research institutions are from Canada, and the largest red institutional collaboration network in the Fig. 5 c indicates that these conditions are consistent with the observation of the largest clustering in the authorship collaboration map originating from Canada.
Journals and publications mapping analysis
Co-citation analysis of journals based on VOSviewer establishes the relationship between items by examining how often they are cited together. Co-citation analysis identifies the connection between two references by counting the number of times they are jointly cited in another article, thereby creating a network of all references within a research area, known as the “intellectual structure [ 25 ]. " This approach is useful for identifying key literature that can help support cross-disciplinary ideas. In the co-citation visualization figures, the size of the circles in the image represents the corresponding TLS values of each entry. Specifically, larger circle sizes indicate higher TLS values [ 33 ].
A total of 78 journal with a minimum number of citations greater than 20 times data points are included in the visualization Fig. 6 . The top five journals with the highest TLS scores are illustrated as follows: JAMA-Journal of The American Medical Association (TLS = 10,969 times), Critical Care Medicine (TLS = 8606 times), New England Journal of Medicine (TLS = 6845 times), Medical Care (TLS = 5010 times) and Annals of Internal Medicine (TLS = 4950 times).

Co-citation analysis of journals on healthcare TQM ( n = 78,1991–2022)
In the green cluster, JAMA and NEJM are the two core publishing medicine journals occupying the most central position, with academic journals on general medicine as the main content of the green cluster, including journals on medical biology and experimental biochemistry.
The blue cluster includes The LANCET and some specific field medicine journals in the small cluster on the right side, as well as journals on healthcare quality and safety and healthcare quality improvement in the upper left corner of the blue cluster.
In the red cluster, the core journals include the Journal of Operation Management, Health Service Research, and Management Science, which focus mainly on the researches on managerial studies and healthcare organizational management.
A total of 55 publications were included in the study with each reference had a minimum number of citations greater than 5 times, and were analyzed using VOSviewer (Fig. 7 ). The top five publications with the highest TLS scores are presented as follows: Shortell SM, 1995, Health Services Research, v30, p377 (TSL = 167 times, cited 37 times), Shortell SM, 2000, Medical Care, v38, p207 (TSL = 104 times, cited 17 times), Berwick DM, 1989, New England Journal of Medicine, v320, p53 (TSL = 64 times, cited 21 times), Deming W.E., 1994, Out Crisis, MIT Press (TSL = 64 times, cited 17times) and Meterko M, 2004, Medical Care, v42, p492 (TSL = 62 times, cited 7 times).Table 1 . below shows the detail of the five highest co-cited publications, including the full titles and corresponding references.
Among the publications, the top one cited, and top one TLS publication was published in 1995, Assessing the impact of continuous quality improvement/total quality management: Concept versus implementation [ 35 ], in which the researchers found that a participative, flexible, risk-taking organizational culture was related to quality improvement implementation. Quality improvement implementation was associated with better patient outcomes and human resource development. Larger hospitals had lower clinical efficiency due to bureaucratic and hierarchical cultures hindering quality improvement. According to the figure, the article most highly co-cited is situated in the central part of the graph, connecting with various clusters, clearly indicating its top position in this field.

Co-cited analysis of reference publications on healthcare TQM ( n = 55,1991–2022)
Co-occurrence mapping analysis
Keywords are an essential part of a research publication and contain the vital information [ 40 ]. Co-occurrence analysis effectively uncovers relationships between research items, especially keywords based on their co-appearance in publications [ 33 ].
A keyword map of TQM in healthcare is presented, where node size signifies frequency and lines between nodes represent co-occurrence [ 22 ].
A total of 1433 keywords were included, and 49 met the criteria of over five occurrences. All keywords were grouped into five clusters (Fig. 8 ): total quality management (in yellow color), quality assurance (in red color), healthcare and performance (in green color), implementation (in blue color), and models (in purple color). The keywords-clustering processing provide insights into the most prominent topics and trending keywords related to total quality management in modern medical services management.
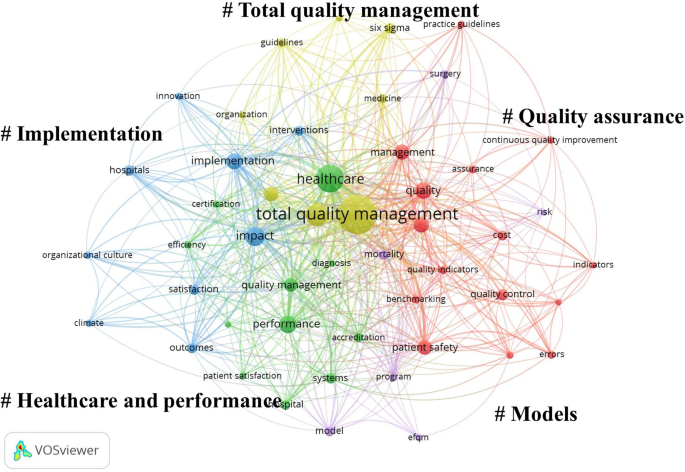
Keywords clustering co-occurrence analysis on healthcare TQM ( n = 49,1991–2022)
In the “total quality management” cluster, other important keywords are “quality improvement”, “quality of healthcare”, and “six sigma”. In the " quality assurance " cluster, other main keywords are “quality”, “patient safety”, “management” and “quality indicators”. The main keywords in “implementation” cluster, others are “impact”, “outcomes”, and “interventions”. The cluster centering keyword “healthcare and performance”, other keywords are “quality management”, “accreditation”, “systems”. The cluster of “models” as its main word, other keywords are “program”, and “EFQM” in purple cluster is an acronym that stands for: European Foundation for Quality Management and its homologous EFQM model, the model is of great importance in the evolvement of quality management theoretical field [ 41 ].
The study will analyze and discuss these different clusters shown in different colors, revealing the distribution of key terms in healthcare TQM research field and the interrelationships among several categories of research focus.
Research hotspots by timeline
VOSviewer assigns a continuous gradient of colors to each keyword based on their chronological appearance in the included publications [ 33 ]. Keywords that appear earlier are assigned darker colors, while keywords that appear later are marked with green, light green, and yellow colors and this research marked the average occurrence year in the development process of this field.

Keyword evolution analysis by year on healthcare TQM ( n = 49,1990–2022)
Figure 9 revealed the development trend of keywords in over the near decades. Prior to the year of 2005, academic research topics and keywords focused mainly on continuous quality improvement, practice guidelines, and assurance.
From 2005 to 2015, research keywords shifted to quality management, quality assurance, healthcare, implementation, impact and a term efqm . And the year of 2010 witnessed the transformation of healthcare TQM from traditional industry TQM concept like six sigma, intervention, etc. to a new concept more suited to the healthcare industry, such as measures of clinical outcomes, patient safety, and safety indicators.
After 2015 and until nowadays, keywords in this field of research changed to organizational performance, efficiency, services safety, systems, and errors. In the recent years, keywords of efficiency and safety climate are trending, takes important places in the field of quality management for a healthcare organization, and they are hotspots in the current studies and academic research [ 42 , 43 ].
These changes in research keywords over time indicate trends and processes of changing directions in this research field at different periods. Meanwhile, recent trends shown in light color suggest that research trends in the other clusters are diversifying, indicating that these research objects’ keywords are changing to varying degrees and have different research priorities.
Global trends in research of TQM in healthcare
Over the past three decades, research directions regarding Total Quality Management in healthcare background have undergone different levels and shifts according to the descriptive statistics for the number of publications by year. The development of this research field is characterized by a wave-like progression. It rapidly developed since 1990, maintained a high plateau from 1993 to 2002, experienced a research downturn from 2003 to 2005. The article “Diagnosing and prognosticating the quality movement – a review on the 25 years quality literature (1987–2011)” in 2013 pointed out that TQM research decreased over time after the 1990s, a phenomenon that was also consistent in the academic research field of healthcare background [ 9 ]. The number of researches of TQM in healthcare backgrounds showed the change with some lags in this study.
From 2006 to 2018, a notable surge be observed in research activity in the field. A second period of rapid development occurred during this time, particularly from 2011 to 2019, reaching its peak in terms of publications in 2018. This phenomenon may indicate a renewed interest and vibrant exploration of the application of TQM in healthcare institutions, also a renewed interest and vitality in the application of TQM in healthcare institutions, which consistent with the observations made by Lee, V-H. and Hew, J-J, this field regained attention and growth since 2013 [ 7 ].
The COVID-19 pandemic have influenced TQM research [ 44 ], there showed a substantial increase in publications in 2022, with 16 papers, compared to previous years implies that continued attention is being paid to the academic research field of TQM in healthcare management.
Observing the rapid increase in the number of annual research paper publications in this field in the three years after the pandemic, and the attainment of higher values than the previous high plateau period, we can reasonably assume that there will be a greater concentration of academic research in the direction of total quality management in healthcare institutions, as well as providing more in-depth knowledge in the future.
Quality and status of global publications
The total number of citations and the H-index are important indicators for reflecting the academic influence and publication quality of a country or a district [ 45 ]. In the field of total quality management research, the United States ranks first in terms of publications, total citations, and H-index, making the greatest contribution in this academic discipline. Italy, the Netherlands, Australia, and England, along with other important developed countries, closely follow, ranking in the top five for publication quantity, citation frequency, and H-index. Additionally, Germany, Canada, and other countries play significant roles in high citation frequencies. The definition of H-index is that a scholar with an index of H has published H publications, each of which has been cited in other publications at least H times [ 46 ], and is often used to measure both the productivity and citation impact of the publications of a scholar [ 45 ].
It is worth mentioning that both China and Taiwan, China have made significant contributions to the relevant academic research. Due to the comprehensiveness and systematization of TQM in the management of healthcare service organizations [ 13 ], developed countries and regions with more advanced healthcare system management models have invested in academic research in this field [ 44 ]. This partially explains the outstanding performance of these regions in the ranking of research in this field.
About journals, Journal of Applied Clinical Medical Physics(IF = 2.1) [ 47 ], Clinical Chemistry and Laboratory Medicine(IF = 6.8) [ 48 ], Accreditation and Quality Assurance (IF = 0.9) [ 49 ], Journal of Continuing Education In The Health Professions(IF = 1.8) [ 50 ], these journals have published a substantial amount of researches on TQM in healthcare background and are considered as the primary sources for future investigation [ 51 , 52 ].
In the institutional publishing rankings, the most important publishing institutions are predominantly from the top five countries with the highest publication output, primarily located in the United States. This demonstrates the significant academic influence of the United States in this field. Additionally, among all the authors, the most prolific contributors in terms of published papers are from the United States. This also indicates that to stay updated with the latest advancements in this field, it is crucial to pay closer attention to the work of these authors and the contributions of the key academic institutions [ 52 ].
Authors, countries or regions, institutions with higher Total Link Strength (TLS) are more likely to collaborate [ 53 , 54 ].The results show that the University of Calgary is the institution with the most published collaborations, and Freniere, Normand, Bissonnette, Jean-Pierre are the most prolific paper collaborators. Friedman, Susan M., Kates, Stephen L., Mendelson, Daniel A. are the authors with the most cited collaborations. Citation analysis calculates the number of times that academic research is cited together, indicating the influence of this research [ 31 ].
The Journal of the American Medical Association (JAMA)(IF = 120.7) [ 55 ] is the most frequently co-cited journal in this field, demonstrating the significant importance and impact of the top general medical journals, with JAMA as a representative, in academic research through their references and citations. Assessing the impact of continuous quality improvement/total quality management: Concept versus implementation [ 35 ] is the most highly cited literature and with other top literatures shown in Table 1 ., which can be considered as milestone or inspiring studies in total quality management research within healthcare services. These highly co-cited literatures are essential references that must be read in this field of research and should be familiar to current and future researchers entering the undergraduate level. Based on them, more comprehensive models or further theoretical studies on healthcare quality management can be developed.
Research focus on TQM in healthcare
This study utilized co-occurrence analysis of keywords to identify three significant research directions. In five clusters: total quality management (yellow cluster), quality assurance (red cluster), implementation (blue cluster), healthcare and performance (green cluster), and models (green cluster), the former four clusters are of large importance in the study. While EFQM, known as European Foundation for Quality Management Excellence Model, is an ever-evolving symbolic model in this field, widely applied in many places [ 41 , 56 , 57 ].
The cluster of “Total Quality Management” includes additional keywords such as “quality improvement”, “quality of healthcare”, and “six sigma”. These keywords are closely associated with total quality management and constitute essential components and aspects of it. Six sigma is a high-level idealistic benchmark for the total quality management for an organization, it takes a vital place. The past decades’ organizational changes, were the main drivers behind the reduction of ICU mortality, as well as reducing cost [ 58 ], the two important parts of quality improvement. Six sigma facilitates waste elimination in production processes in hospitals. The continuous improvement model integrates practical knowledge with an understanding of the system under improvement, utilizing observations and modifications to enable measurable outcomes. Six Sigma can also increase the effectiveness of evidence-based quality improvement programs in hospitals [ 59 ].
Within the second cluster of “quality assurance,” there are other significant keywords such as “quality”, “patient safety”, “management” and “quality indicators”. These keywords collectively emphasize assuring healthcare safety, which is fundamental for the quality of healthcare. The key to total quality management in healthcare is assuring patient safety, which requires monitoring and measuring patient safety using appropriate health quality indicators. The core is to achieve a patient orientated quality assurance related to the medical benefits and the patient individual needs [ 60 ].Most quality assuring plans are related to the development and evaluation of quality indicators, and it is useful to have a broader reflection on the concept of indicators. In healthcare field, the specific design of a patient safety indicator system depends on the precise definition and measurement context, while in total quality management, the establishment of such an indicator system requires the participation of all staff members in hospitals [ 61 ].
In the “implementation” cluster, other major keywords include “impact”, “outcomes”, and “interventions”. The implementation of TQM principles in the healthcare field, requires a radical change in management practice and the organizational culture and its philosophy to effectively increase healthcare performance [ 62 ]. A healthcare management conceptual framework from TQM noticed that some characteristics of organizational culture are associated with error reduction, to improve the medical outcomes [ 63 ].And clinical interventions conducted under the conception of TQM proved effective in studies [ 64 , 65 ].
The cluster centers on the keyword “healthcare and performance”, with other keywords including “quality management”, “accreditation”, and “systems”. While there are contradictory findings about the impact of accreditation on improving the quality of healthcare services, accreditation continues to be recognized internationally as a quality assurance tool [ 66 ]. Accreditation within the healthcare sector constitutes a pivotal element in TQM research that is centered on the realm of healthcare, with a substantial corpus of literature devoted to investigating this domain. Notably, despite the endeavors of certain studies, including a comprehensive one encompassing 36,777 patients, no discernible correlation has been discerned between the accredited status of hospitals and patient satisfaction [ 67 ]. Both clinical medical and laboratory certification systems are conducive to building higher-quality healthcare services in any country or region, enhancing the performance of healthcare [ 68 , 69 ].
Comparison with previous studies
As V-H. Lee and J-J. Hew pointed out in their 2017 paper, the number of citations received by TQM articles published between 2006 and 2017 increased year by year, indicating that it remains a prominent and popular research topic, and encouraging subsequent scholars to strive to advance TQM research to higher levels. They also suggested that scholars consider conducting cross-national comparisons between developed and developing countries and combining TQM with other research fields [ 7 ].
Accordingly, the bibliometric research for the time period of 1991–2022 in this article found that developed countries such as the USA and European nations lead in terms of historical total publications. The only non-developed country to make it into the top ten is China. Additionally, this article focuses on a bibliometric study of TQM in the context of health services, which refines the research objectives outlined in previous studies.
Majdi M Alzoubi, K S Hayati et al. conducted a meta-analysis of 25 of the most relevant TQM articles in healthcare between 2005 and 2016, which showed that there are relatively few studies on TQM in the healthcare context. They also believe that such TQM healthcare research is valuable for both researchers and healthcare managers. They identified key terms in TQM for healthcare, including education and training, continuous quality improvement, customer focus/satisfaction, top management commitment, and teamwork, but did not analyze the evolution of these themes over time [ 13 ]. This study, however, uses bibliometric methods to provide a comprehensive analysis of the changes in TQM keywords in healthcare from 1991 to 2022. While systematic reviews focus on detailed content analysis, this bibliometric study takes a broader perspective to investigate the field, providing less granular detail but examining different aspects of the topic.
Chen Zhang led and conducted a bibliometric study on the application of TQM in the service industry, analyzing 3,774 English research articles published between 2004 and 2017. They performed keyword mapping and extensive co-citation analysis. The contribution of this study lies in better understanding the obstacles and difficulties hindering the successful application of TQM in the service sector. It also addresses the limitation of most TQM empirical studies being case studies, which lack interconnection, making generalization impractical.
In contrast, this article primarily focuses on high-quality database articles, specifically 326 articles retrieved from the Web of Science Core Collection, which are more representative and influential, effectively selecting the best of the available literature. Furthermore, it provides a more detailed discussion and analysis of the keywords. The time range of this study is broader than previous studies, covering the period from 1991 to 2022.
Significance and future directions
This study conducted a bibliometric analysis of high-quality English research articles on Total Quality Management (TQM) in the healthcare context, spanning the period from 1991 to 2022. Drawing from an overview of English literature and mapping results retrieved from the Web of Science (WOS) core collection, we identified that global healthcare research has centered on organizational transformation, encompassing the evolving trends in management practices and philosophies aligned with TQM concepts. Enhancing the quality management climate in healthcare and reducing errors emerged as central themes. Looking ahead, TQM is expected to play an even more pivotal role in the intricate landscape of healthcare quality management practices, with more researches on refined TQM-based indicators anticipated.
In future studies, the focus will be as follows. Healthcare quality indicators are measurable, objective, and quantitative measures of key system elements’ performance in TQM, which reflect to what extent a system meets the needs and expectations of its customers [ 70 ].In TQIP, a regional quality indicator project in Asia, indicators measures the healthcare quality of hospitals, confirming that the enhancement in both quality and relative efficiency coincides with the philosophy of total quality management [ 71 ], which inspires the future TQM researches.
Recent research has focused on keywords such as error, hospital quality climate, and TQM models, in addition to indicators analysis. Future research may further develop on these foundations. The carrying out of TQM in clinical practices, in the near future, can definitely enhance compliance and control in the context of a highly intricate care unit in hospitals, and TQM clinical indicator is a replicable and scalable quality assessment approach for a complex clinical setting, cutting medical errors and assuring the safety of healthcare services [ 72 ].
Strength and limitations
This article is the first to employ bibliometric methods to investigate the implementation of Total Quality Management (TQM) in healthcare in such an elaborate manner, in such a timeframe, and to such an extent. By utilizing descriptive statistics, visualization software and bibliometric analysis, the study aims to comprehensively understand the current state and future trends in this field and identify hotspots and collaboration relationships among countries, authors, and institutions.
However, the study has several limitations. While several large academic databases currently exist, considering the accessibility and quality of the contents of the collected databases, only core collection of WOS database of academic articles was adopted as the source of materials, and it is possible that some high-quality research articles were omitted with the WOS core collection’s criteria [ 73 ]. It is worth noting that while a single database with well-defined search criteria is often sufficient for rigorous bibliometric studies, and the WOS Core Collection includes all high-quality journals and papers under the Clarivate’s standards (SCI, SSCI, etc.), the potential for omissions in terms of the completeness of high-quality papers across different databases remains one of the limitations [ 74 , 75 ].
Secondly, all publications including in this article are in English, which may overlook relevant articles published in other languages. Thirdly, there can be inherent differences between bibliometric analysis results and actual current research outcomes. For example, some high-quality but low-cited-numbered recent publications (by the time of September,2023) might not have received sufficient attention. Lastly, biases may exist due to variations in author names and keyword expressions.
This article reveals the research themes and trends related to TQM in the healthcare background using bibliometric methods. It describes that the research field of TQM is still led by the USA and European countries, and discovers that Canadian research institutions have the largest cross-institutional cooperation and scholar groups. It also finds that a paper by Shortell et al. in 1995 has the highest number of co-citations in this field, and that world-renowned medical, biochemical, and management journals have provided extensive interdisciplinary evidence for TQM research in healthcare. Based on mapping, this study identifies six major clusters of TQM medical research, discovers that quality management and TQM implementation are the main topics that have already been studied, and finds that the latest focus is on hospital quality management models such as EFQM and indicator systems, which may also shift to research on quality climate researches. With the observation of a fluctuating upward trend, we believe that this field will produce more high-quality English papers in the future.
Data availability
The data supporting the findings of this study are available in web of science database as described in the Methods section of the manuscript.
Al-Shdaifat EA. Implementation of total quality management in hospitals. J Taibah Univ Med Sci. 2015;10(4):461–6.
Google Scholar
Aquilani B, et al. A systematic literature review on total quality management critical success factors and the identification of new avenues of research. TQM J. 2017;29(1):184–213.
Article Google Scholar
Rokke C, Yadav OP. Challenges and barriers to total quality management: an overview. Int J Perform Eng. 2012;8:653–65.
Miller WJ. A working definition for total quality management (TQM) researchers. J Qual Manage. 1996;1(2):149–59.
Gardner DB, Cummings C. Total quality management and shared governance: synergistic processes. Nurs Adm Q. 1994;18(4):56–64.
Article PubMed CAS Google Scholar
Sadikoglu E, Zehir C. Investigating the effects of innovation and employee performance on the relationship between total quality management practices and firm performance: an empirical study of Turkish firms. Int J Prod Econ. 2010;127(1):13–26.
Lee V, Hew J. Is TQM fading away? A bibliometric analysis of a decade (2006–2015). Int J Serv Econ Manage. 2017;8:227.
Agus A. TQM as a Focus for Improving Overall Service Performance and customer satisfaction: an empirical study on a Public Service Sector in Malaysia. Total Qual Manage Bus Excellence. 2004;15(5–6):615–28.
Dahlgaard-Park SM, et al. Diagnosing and prognosticating the quality movement - a review on the 25 years quality literature (1987–2011). Total Qual Manage Bus Excellence. 2013;24(1–2):1–18.
Prajogo D. The comparative analysis of TQM practices and quality performance between manufacturing and service firms. Int J Serv Ind Manag. 2005;16:217–28.
Chang T-H, Fuzzy VIKOR. Method: a case study of the hospital service evaluation in Taiwan. Inf Sci. 2014;271:196–212.
Getele GK, Jean AT. Total quality management in the healthcare sector: an empirical research from Ethiopia. Hum Syst Manage. 2020;39:441–53.
Alzoubi MM, et al. Total quality management in the health-care context: integrating the literature and directing future research. Risk Manage Healthc Policy. 2019;12:167–77.
AlQahtani J, Turky E, Al-Ghamdi A. CONTINUOUS IMPROVEMENT IN TQM. Int J Manage Inform Technol. 2014;9:1718–22.
Durairatnam S, Chong S-C, Jusoh M, People-Related TQM. Practices, Organisational Culture, Organisational Justice and Employee Work-related Attitudes for Quality Performance: A Research Agenda. 2019.
Yousef N, Yousef F. Using total quality management approach to improve patient safety by preventing medication error incidences. BMC Health Serv Res, 2017. 17.
Hidayah N, Arbianingsih, Ilham. The impact of integrated quality management-based health services on general hospital quality. 2022. 10.
Aggarwal A, Aeran H, Rathee M. Quality management in healthcare: the pivotal desideratum. J Oral Biol Craniofac Res. 2019;9(2):180–2.
Article PubMed Google Scholar
Zhang C, Moreira MRA, Sousa PSA. A bibliometric view on the use of total quality management in services. Total Qual Manage Bus Excellence. 2021;32(13–14):1466–93.
Web of Science Science Citation Index Expanded (SCIE) . 2023; https://clarivate.com.com/solutions/web-of-science/
Singh VK, et al. The journal coverage of web of Science, Scopus and dimensions: a comparative analysis. Scientometrics. 2021;126(6):5113–42.
Qin X et al. Organisational Culture Research in Healthcare: A Big Data Bibliometric Study. Healthc (Basel), 2023. 11(2).
Stapor K, Descriptive and Inferential Statistics, in Introduction to Probabilistic and Statistical Methods with Examples in R, Stapor K. Editor. 2020, Springer International Publishing: Cham. pp. 63–131.
Okubo Y. Bibliometric indicators and analysis of research systems: methods and examples. 1997.
Zupic I, Čater T. Bibliometric methods in management and Organization. Organizational Res Methods. 2014;18:429–72.
Cobo MJ, et al. Science mapping software tools: review, analysis, and cooperative study among tools. J Am Soc Inform Sci Technol. 2011;62(7):1382–402.
van Eck NJ, et al. A comparison of two techniques for bibliometric mapping: multidimensional scaling and VOS. J Am Soc Inform Sci Technol. 2010;61(12):2405–16.
Kirby A. Exploratory bibliometrics: using VOSviewer as a preliminary Research Tool. Publications. 2023;11. https://doi.org/10.3390/publications11010010 .
Arruda H, et al. VOSviewer and Bibliometrix. J Med Libr Assoc. 2022;110(3):392–5.
Article PubMed PubMed Central Google Scholar
van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84(2):523–38.
Donthu N, et al. How to conduct a bibliometric analysis: an overview and guidelines. J Bus Res. 2021;133:285–96.
Shao B, Li X, Bian G. A survey of research hotspots and frontier trends of recommendation systems from the perspective of knowledge graph. Expert Syst Appl. 2021;165:113764.
Waltman NJvEL. VOSviewer Manual . 2023 [cited 2023; https://www.vosviewer.com/documentation/Manual_VOSviewer_1.6.19.pdf
Reyes-Gonzalez L, Gonzalez-Brambila CN, Veloso F. Using co-authorship and citation analysis to identify research groups: a new way to assess performance. Scientometrics. 2016;108(3):1171–91.
Shortell SM, et al. Assessing the impact of continuous quality improvement/total quality management: concept versus implementation. Health Serv Res. 1995;30(2):377–401.
PubMed PubMed Central CAS Google Scholar
Shortell SM, et al. Assessing the impact of total quality management and organizational culture on multiple outcomes of care for coronary artery bypass graft surgery patients. Med Care. 2000;38(2):207–17.
Berwick DM. Continuous improvement as an ideal in health care. N Engl J Med. 1989;320(1):53–6.
Deming WE. Out of the Crisis . 1994.
Meterko M, Mohr DC, Young GJ. Teamwork culture and patient satisfaction in hospitals. Med Care. 2004;42(5):492–8.
Wang H, et al. A historical review and bibliometric analysis of GPS research from 1991–2010. Scientometrics. 2013;95(1):35–44.
Management EF. f.Q. European Foundation for Quality Management http://www.efqm.org
Hartmann CW, et al. Relationship of Hospital Organizational Culture to Patient Safety Climate in the Veterans Health Administration. Med Care Res Rev. 2009;66(3):320–38.
Speroff T, et al. Organisational culture: variation across hospitals and connection to patient safety climate. Qual Saf Health Care. 2010;19(6):592–6.
PubMed CAS Google Scholar
Xiao A, et al. Emerging research trends of total quality management in the COVID-19 pandemic: a dynamic evolution analysis. Economic Research-Ekonomska Istraživanja. 2023;36(2):2140305.
Alonso S, et al. h-Index: a review focused in its variants, computation and standardization for different scientific fields. J Informetrics. 2009;3(4):273–89.
Hirsch JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci U S A. 2005;102(46):16569–72.
Article PubMed PubMed Central CAS Google Scholar
Medicine AA. o.P.i. Journal Metrics: Journal of Applied Clinical Medical Physics . 2022 [cited 2023; https://aapm.onlinelibrary.wiley.com/page/journal/15269914/journal-metrics
CCLM. Clinical Chemistry and Laboratory Medicine (CCLM) IF = 6.8 . CLM is the official journal of the European Federation of Clinical Chemistry and Laboratory Medicine 2023 [cited 2023; https://www.degruyter.com/journal/key/cclm/html
Accreditation and Quality Assurance Journal for Quality, Comparability and Reliability in Chemical Measurement. 2023 [cited 2023; https://www.springer.com/journal/769
Tanno LK, Demoly P. Preparing international classification of diseases (ICD)-11: model of allergic and hypersensitivity conditions. Revue Francaise D Allergologie. 2020;60(8):595–9.
Aksnes DW, Langfeldt L, Wouters P. Citations, citation indicators, and Research Quality: an overview of Basic concepts and theories. Volume 9. SAGE Open; 2019. p. 2158244019829575. 1.
Carpenter CR, Cone DC, Sarli CC. Using publication metrics to highlight academic productivity and research impact. Acad Emerg Med. 2014;21(10):1160–72.
Fonseca BdPFe, et al. Co-authorship network analysis in health research: method and potential use. Health Res Policy Syst. 2016;14(1):34.
Mao X et al. The status and trends of coronavirus research: a global bibliometric and visualized analysis. Medicine, 2020. 99(22).
Epstein RH, Dexter F. Rescheduling of previously cancelled Surgical cases does not increase variability in operating Room Workload when cases are scheduled based on maximizing efficiency of Use of operating Room Time. Anesth Analg. 2013;117(4):995–1002.
Gómez JG, Martínez M, Costa, Martínez Lorente ÁR. EFQM Excellence Model and TQM: an empirical comparison. Total Qual Manage Bus Excellence. 2017;28(1–2):88–103.
Calvo-Mora A, Domínguez-Cc M, Criado F. Assessment and improvement of organisational social impact through the EFQM Excellence Model. Total Qual Manage Bus Excellence. 2018;29(11–12):1259–78.
van der Sluijs AF et al. The impact of changes in intensive care organization on patient outcome and cost-effectiveness-a narrative review. J Intensive Care, 2017. 5.
Lavin P, Vetter MJ. Using lean six Sigma to increase the effectiveness of an evidence-based quality improvement program. J Nurs Care Qual. 2022;37(1):81–6.
Otto V, Zenker W. From quality assurance to total quality management. Experiences in quality assurance. Zentralbl Chir. 2000;125:137–40.
PubMed Google Scholar
Quentin W, Brownwood PV. In: Busse KN, Panteli R D, et al. editors. I, et al, Measuring healthcare quality., in improving healthcare quality in Europe: characteristics, effectiveness and implementation of different strategies. Health Policy Series. Copenhagen (Denmark): European Observatory on Health Systems and Policies; 2019.
Gregori D, et al. Knowledge, practice and faith on total Quality Management principles among workers in the Health Care System: evidence from an Italian investigation. J Eval Clin Pract. 2009;15(1):69–75.
Stock GN, McFadden KL, Gowen CR. Organizational culture, critical success factors, and the reduction of hospital errors. Int J Prod Econ. 2007;106(2):368–92.
Seltzer SE, et al. Expediting the turnaround of radiology reports in a teaching hospital setting. Am J Roentgenol. 1997;168(4):889–93.
Article CAS Google Scholar
Isouard G. A quality management intervention to improve clinical laboratory use in acute myocardial infarction. Med J Aust. 1999;170(1):11–4.
Alhawajreh MJ, Paterson AS, Jackson WJ. Impact of hospital accreditation on quality improvement in healthcare: a systematic review. PLoS ONE. 2023;18(12):e0294180.
Sack C, et al. Is there an association between hospital accreditation and patient satisfaction with hospital care? A survey of 37 000 patients treated by 73 hospitals. Int J Qual Health Care. 2011;23(3):278–83.
Ehrmeyer SS, Laessig RH. The American (USA) perspective six years after implementation of CLIA’88 (Federal) regulations. Accred Qual Assur. 1999;4(3):93–8.
Goldschmidt HMJ, van der Weide WE, van Gennip E. Application of the NIAZ frame of reference; impact on a departmental level. Accred Qual Assur. 2001;6(9–10):431–4.
Simundic AM, Topic E. Quality indicators. Biochemia Med. 2008;18(3):311–9.
Chang SJ, et al. Taiwan quality indicator project and hospital productivity growth. Omega-International J Manage Sci. 2011;39(1):14–22.
Sussmane JB, Torbati D, Gitlow HS. Measuring the quality of therapeutic apheresis care in the pediatric intensive care unit. J Clin Apheresis. 2012;27(2):43–50.
Clarivate. Web of Science Core Collection Overview . 2021 [cited 2024; v2.0:[ https://webofscience.help.clarivate.com/Content/wos-core-collection/wos-core-collection.htm
Romanelli JP, et al. Four challenges when conducting bibliometric reviews and how to deal with them. Environ Sci Pollut Res. 2021;28(43):60448–58.
AlRyalat SAS, Malkawi LW, Momani SM. Comparing Bibliometric Analysis using PubMed, Scopus, and web of Science Databases. J Vis Exp, 2019(152).
Download references
Acknowledgements
We would like to extend our sincere gratitude to Chinese Academy of Medical Sciences and Peking Union Medical College for providing the supportive environment and necessary facilities that facilitated the writing of this manuscript. Their commitment to research excellence has been instrumental in the completion of this work.
This research was supported by Chinese Academy of Medical Sciences and Peking Union Medical College, China (Grant number: 2021-RC630-001), and the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences-A Strategic Study on Healthy China Development and Health System Reform (2021-I2M-1-046).
Author information
Zhiyuan Hu and Richard Szewei Wang contributed equally to this work and share first authorship.
Yuanli Liu, Herng-Chia Chiu, and Bing-Long Wang contributed equally to this work and share corresponding authorship.
Authors and Affiliations
School of Health Policy and Management, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, 100730, China
Zhiyuan Hu, Xiaoping Qin, Yuanli Liu & Bing-Long Wang
Tsinghua-Berkeley Shenzhen Institute, Tsinghua University, Shenzhen, 518055, China
Richard Szewei Wang
College of Medical and Health Science, Asia University, Taichung, 41354, Taiwan
Yu-Ni Huang
Institute for Hospital Management, Tsinghua University, Shenzhen, 518017, China
Herng-Chia Chiu
Bloomberg School of Public Health, Johns Hopkins University, Baltimore, 21218, USA
You can also search for this author in PubMed Google Scholar
Contributions
Z.H. and R.W. did the acquisition and interpretation of data and wrote the main manuscript text and Z.H. prepared all figures and tables. X.Q, Y.H., L.L., and B.W. designed the work. H.C., X.Q, Y.H., L.L., Y.L. and B.W. substantively revised the manuscript. B.W. and Y.L. obtained the funding support for the manuscript and publication. All authors substantively reviewed the manuscript.
Corresponding authors
Correspondence to Herng-Chia Chiu , Yuanli Liu or Bing-Long Wang .
Ethics declarations
Ethics approval and consent to participate.
There are no human or animal studies in this manuscript, and no potentially identifiable human images or data is presented in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Hu, Z., Wang, R.S., Qin, X. et al. The global research landscape and future trends in healthcare Total Quality Management. Arch Public Health 82 , 193 (2024). https://doi.org/10.1186/s13690-024-01420-3
Download citation
Received : 26 March 2024
Accepted : 12 October 2024
Published : 28 October 2024
DOI : https://doi.org/10.1186/s13690-024-01420-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Total quality management
- Healthcare management
- Bibliometric study
Archives of Public Health
ISSN: 2049-3258
- General enquiries: [email protected]
Artificial Intelligence Applications in Quality Management System: A Bibliometric Study
Ieee account.
- Change Username/Password
- Update Address
Purchase Details
- Payment Options
- Order History
- View Purchased Documents
Profile Information
- Communications Preferences
- Profession and Education
- Technical Interests
- US & Canada: +1 800 678 4333
- Worldwide: +1 732 981 0060
- Contact & Support
- About IEEE Xplore
- Accessibility
- Terms of Use
- Nondiscrimination Policy
- Privacy & Opting Out of Cookies
A not-for-profit organization, IEEE is the world's largest technical professional organization dedicated to advancing technology for the benefit of humanity. © Copyright 2024 IEEE - All rights reserved. Use of this web site signifies your agreement to the terms and conditions.
Advertisement
From total quality management to Quality 4.0: A systematic literature review and future research agenda
- Review Article
- Open access
- Published: 13 March 2023
- Volume 10 , pages 191–205, ( 2023 )
Cite this article
You have full access to this open access article

- Hu-Chen Liu 1 ,
- Ran Liu 1 ,
- Xiuzhu Gu 2 &
- Miying Yang 3
6980 Accesses
16 Citations
Explore all metrics
Quality 4.0 is an emerging concept that has been increasingly appreciated because of the intensification of competition, continually changing customer requirements and technological evolution. It deals with aligning quality management practices with the emergent capabilities of Industry 4.0 to improve cost, time, and efficiency and increase product quality. This article aims to comprehensively review extant studies related to Quality 4.0 to uncover current research trends, distil key research topics, and identify areas for future research. Thus, 46 journal articles extracted from the Scopus database from 2017 to 2022 were collected and reviewed. A descriptive analysis was first performed according to the year-wise publication, sources of publication, and research methods. Then, the selected articles were analyzed and classified according to four research themes: Quality 4.0 concept, Quality 4.0 implementation, quality management in Quality 4.0, and Quality 4.0 model and application. By extracting the literature review findings, we identify the Quality 4.0 definitions and features, develop the quality curve theory, and highlight future research opportunities. This study supports practitioners, managers, and academicians in effectively recognizing and applying Quality 4.0 to enhance customer satisfaction, achieve innovation enterprise efficiency, and increase organizational competitiveness in the era of Industry 4.0.

Article PDF
Download to read the full article text
Similar content being viewed by others

Study and Review of Quality 4.0 in the Industry

Illustrating the Development of Quality Management Instrumentation: A Systematic Literature Review

Important Parameters Influencing Total Quality Management: A Comparative Study
Avoid common mistakes on your manuscript.
Ali K, Johl S K (2022). Soft and hard TQM practices: Future research agenda for Industry 4.0. Total Quality Management & Business Excellence, 33(13–14): 1625–1655
Article Google Scholar
Alzahrani B, Bahaitham H, Andejany M, Elshennawy A (2021). How ready is higher education for Quality 4.0 transformation according to the LNS research framework? Sustainability, 13(9): 5169
Antony J, McDermott O, Sony M (2022a). Quality 4.0 conceptualisation and theoretical understanding: A global exploratory qualitative study. TQM Journal, 34(5): 1169–1188
Antony J, Sony M, Furterer S, McDermott O, Pepper M (2022b). Quality 4.0 and its impact on organizational performance: An integrative viewpoint. TQM Journal, 34(6): 2069–2084
Antony J, Sony M, McDermott O, Jayaraman R, Flynn D (2023). An exploration of organizational readiness factors for Quality 4.0: An intercontinental study and future research directions. International Journal of Quality & Reliability Management, 40(2): 582–606
Asif M (2020). Are QM models aligned with Industry 4.0? A perspective on current practices. Journal of Cleaner Production, 258: 120820
Balouei Jamkhaneh H, Shahin A, Parkouhi S V, Shahin R (2022). The new concept of quality in the digital era: A human resource empowerment perspective. TQM Journal, 34(1): 125–144
Baran E, Korkusuz Polat T (2022). Classification of Industry 4.0 for total quality management: A review. Sustainability, 14(6): 3329
Broday E E (2022). The evolution of quality: From inspection to Quality 4.0. International Journal of Quality and Service Sciences, 14(3): 368–382
Bui L T C, Carvalho M, Pham H T, Nguyen T T B, Duong A T B, Truong Quang H (2022). Supply chain quality management 4.0: Conceptual and maturity frameworks. International Journal of Quality & Reliability Management, in press, doi: https://doi.org/10.1108/IJQRM-07-2021-0251
Chiarini A (2020). Industry 4.0, quality management and TQM world: A systematic literature review and a proposed agenda for further research. TQM Journal, 32(4): 603–616
Chiarini A, Kumar M (2022). What is Quality 4.0? An exploratory sequential mixed methods study of Italian manufacturing companies. International Journal of Production Research, 60(16): 4890–4910
Christou I T, Kefalakis N, Soldatos J K, Despotopoulou A M (2022). End-to-end industrial IoT platform for Quality 4.0 applications. Computers in Industry, 137: 103591
Clancy R, Bruton K, O’Sullivan D T J, Cloonan A J (2022). The HyDAPI framework: A versatile tool integrating Lean Six Sigma and digitalisation for improved quality management in Industry 4.0. International Journal of Lean Six Sigma, in press, doi: https://doi.org/10.1108/IJLSS-12-2021-0214
Di Vaio A, Hassan R, Alavoine C (2022). Data intelligence and analytics: A bibliometric analysis of human-artificial intelligence in public sector decision-making effectiveness. Technological Forecasting and Social Change, 174: 121201
Dias A M, Carvalho A M, Sampaio P (2022). Quality 4.0: Literature review analysis, definition and impacts of the digital transformation process on quality. International Journal of Quality & Reliability Management, 39(6): 1312–1335
Elibal K, Özceylan E (2022). Comparing Industry 4.0 maturity models in the perspective of TQM principles using Fuzzy MCDM methods. Technological Forecasting and Social Change, 175: 121379
Emblemsvåg J (2020). On Quality 4.0 in project-based industries. TQM Journal, 32(4): 725–739
Escobar C A, McGovern M E, Morales-Menendez R (2021). Quality 4.0: A review of big data challenges in manufacturing. Journal of Intelligent Manufacturing, 32(8): 2319–2334
Fonseca L, Amaral A, Oliveira J (2021). Quality 4.0: The EFQM 2020 model and Industry 4.0 relationships and implications. Sustainability, 13(6): 3107
Gebhardt M, Kopyto M, Birkel H, Hartmann E (2022). Industry 4.0 technologies as enablers of collaboration in circular supply chains: A systematic literature review. International Journal of Production Research, 60(23): 6967–6995
Glogovac M, Ruso J, Maricic M (2022). ISO 9004 maturity model for quality in Industry 4.0. Total Quality Management & Business Excellence, 33(5–6): 529–547
Hyun Park S, Seon Shin W, Hyun Park Y, Lee Y (2017). Building a new culture for quality management in the era of the Fourth Industrial Revolution. Total Quality Management & Business Excellence, 28(9–10): 934–945
Jacob D (2017). What is Quality 4.0? Online Blog
Kannan K S P N, Garad A (2021). Competencies of quality professionals in the era of Industry 4.0: A case study of electronics manufacturer from Malaysia. International Journal of Quality & Reliability Management, 38(3): 839–871
Maganga D P, Taifa I W R (2022). Quality 4.0 transition framework for Tanzanian manufacturing industries. TQM Journal, in press, doi: https://doi.org/10.1108/TQM-01-2022-0036
Maganga D P, Taifa I W R (2023). Quality 4.0 conceptualisation: An emerging quality management concept for manufacturing industries. TQM Journal, 35(2): 389–413
Nenadál J, Vykydal D, Halfarová P, Tylečková E (2022). Quality 4.0 maturity assessment in light of the current situation in the Czech Republic. Sustainability, 14(12): 7519
Prashar A (2022). Quality management in Industry 4.0 environment: A morphological analysis and research agenda. International Journal of Quality & Reliability Management, in press, doi: 10.1108/IJQRM-10-2021-0348
Ramezani J, Jassbi J (2020). Quality 4.0 in action: Smart hybrid fault diagnosis system in plaster production. Processes, 8(6): 634
Ranjith Kumar R, Ganesh L S, Rajendran C (2022). Quality 4.0: A review of and framework for quality management in the digital era. International Journal of Quality & Reliability Management, 39(6): 1385–1411
Sader S, Husti I, Daróczi M (2019). Industry 4.0 as a key enabler toward successful implementation of total quality management practices. Periodica Polytechnica Social and Management Sciences, 27(2): 131–140
Sader S, Husti I, Daroczi M (2022). A review of Quality 4.0: Definitions, features, technologies, applications, and challenges. Total Quality Management & Business Excellence, 33(9–10): 1164–1182
Saihi A, Awad M, Ben-Daya M (2023). Quality 4.0: Leveraging Industry 4.0 technologies to improve quality management practices, a systematic review. International Journal of Quality & Reliability Management, 40(2): 628–650
Santos G, Sá J C, Félix M J, Barreto L, Carvalho F, Doiro M, Zgodavová K, Stefanović M (2021). New needed quality management skills for quality managers 4.0. Sustainability, 13(11): 6149
Sariyer G, Mangla S K, Kazancoglu Y, Ocal Tasar C, Luthra S (2021). Data analytics for quality management in Industry 4.0 from an MSME perspective. Annals of Operations Research, in press, doi: https://doi.org/10.1007/s10479-021-04215-9
Silva C S, Borges A F, Magano J (2022). Quality Control 4.0: A way to improve the quality performance and engage shop floor operators. International Journal of Quality & Reliability Management, 39(6): 1471–1487
Singh J, Ahuja I P S, Singh H, Singh A (2022). Development and implementation of Autonomous Quality Management System (AQMS) in an automotive manufacturing using Quality 4.0 concept: A case study. Computers & Industrial Engineering, 168: 108121
Sony M, Antony J, Douglas J A (2020). Essential ingredients for the implementation of Quality 4.0: A narrative review of literature and future directions for research. TQM Journal, 32(4): 779–793
Sony M, Antony J, Douglas J A, McDermott O (2021). Motivations, barriers and readiness factors for Quality 4.0 implementation: An exploratory study. TQM Journal, 33(6): 1502–1515
Souza F F, Corsi A, Pagani R N, Balbinotti G, Kovaleski J L (2022). Total quality management 4.0: Adapting quality management to Industry 4.0. TQM Journal, 34(4): 749–769
Sozinova A A, Saveleva N K (2022). Marketing quality management in Industry 4.0 in transborder markets. International Journal of Qualitative Research, 16(3): 955–968
Sureshchandar G S (2022). Quality 4.0: Understanding the criticality of the dimensions using the analytic hierarchy process (AHP) technique. International Journal of Quality & Reliability Management, 39(6): 1336–1367
Sureshchandar G S (2023). Quality 4.0: A measurement model using the confirmatory factor analysis (CFA) approach. International Journal of Quality & Reliability Management, 40(1): 280–303
Tambare P, Meshram C, Lee C C, Ramteke R J, Imoize A L (2022). Performance measurement system and quality management in data-driven Industry 4.0: A review. Sensors, 22(1): 224
Tan T, Mills G, Papadonikolaki E, Liu Z (2021). Combining multi-criteria decision making (MCDM) methods with building information modelling (BIM): A review. Automation in Construction, 121: 103451
Thekkoote R (2022). Enabler toward successful implementation of Quality 4.0 in digital transformation era: A comprehensive review and future research agenda. International Journal of Quality & Reliability Management, 39(6): 1368–1384
Yadav N, Shankar R, Singh S P (2021). Critical success factors for Lean Six Sigma in Quality 4.0. International Journal of Quality and Service Sciences, 13(1): 123–156
Zheng T, Ardolino M, Bacchetti A, Perona M (2021). The applications of Industry 4.0 technologies in manufacturing context: A systematic literature review. International Journal of Production Research, 59(6): 1922–1954
Zonnenshain A, Kenett R S (2020). Quality 4.0: The challenging future of quality engineering. Quality Engineering, 32(4): 614–626
Download references
Author information
Authors and affiliations.
School of Economics and Management, Tongji University, Shanghai, 200092, China
Hu-Chen Liu & Ran Liu
Department of Industrial Engineering and Economics, Tokyo Institute of Technology, Tokyo, 152-8552, Japan
Group of Sustainability, School of Management, Cranfield University, Cranfield, MK43 0AL, UK
Miying Yang
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Ran Liu .
Additional information
This work was partially supported by the major project of National Social Science Fund of China (Grant No. 21ZDA024).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Liu, HC., Liu, R., Gu, X. et al. From total quality management to Quality 4.0: A systematic literature review and future research agenda. Front. Eng. Manag. 10 , 191–205 (2023). https://doi.org/10.1007/s42524-022-0243-z
Download citation
Received : 31 July 2022
Accepted : 01 November 2022
Published : 13 March 2023
Issue Date : June 2023
DOI : https://doi.org/10.1007/s42524-022-0243-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- quality management
- Quality 4.0
- Industry 4.0
- literature review
- predictive quality
- Find a journal
- Publish with us
- Track your research
quality management systems Recently Published Documents
Total documents.
- Latest Documents
- Most Cited Documents
- Contributed Authors
- Related Sources
- Related Keywords
Requirements for Certification of Aviation, Space, and Defense Quality Management Systems
Developing a quality management systems framework for business management institutes.
There has been an increasing awareness about implementation of quality management systems (QMS) in higher educational institutions worldwide in the recent past. Improved awareness levels among aspiring students, competitive environment, students' preference to seek admission in quality-oriented institutions, compelling norms of regulatory authorities, and the urge of institutions to be among the top are the major reasons for this increased awareness. Existence and continuance of some of the institutes, especially the management institutes, has become a challenging task without focusing on QMS through a strategic approach. Implementation of QMS provides a direction for accreditations by top-rated agencies. This chapter develops a framework to enable the implementation of QMS by the management institutes by conducting a systematic analysis of accreditation standards of various agencies worldwide. The outcomes of this research would enable the management and other stakeholders of business management institutes to focus on key aspects towards implementing the QMS.
Establishing Knowledge Management Model of Quality Management Systems for Higher Education Institutions
Knowledge management is a valuable antecedent in enhancing the competitive advantage and sustainability of an organization. Various quality management standards acknowledge the necessities and importance of knowledge management within quality management since both areas complement each other. Therefore, this state drives the need to conduct more knowledge management research related to quality management. However, research on knowledge management within quality management systems specifically in higher education institutions is still underexplored. This chapter attempts to explore the establishment of a knowledge management model in supporting the implementation of quality management standards. Several knowledge management enabler factors are suggested, namely strategic focus and leadership, culture, organization, structure, technology, processes, top management, measurement, knowledge, and people. An empirical study is suggested in the future to further support the conceptual knowledge management model established in this chapter.
Food Microbial Hazards, Safety, and Quality Control
Food is any material or substance eaten or drunk to provide energy and nutrients for the body's growth, development, and maintenance. Food can be considered safe if it is free from all hazardous substances that can affect consumer health. Food safety issues can place a high burden of responsibility on traders, government bodies, and international organizations. This chapter covers the hazards, their types, foodborne diseases, and strategies to ensure food safety and quality. Different food quality and safety assurance programs are discussed as well like quality management systems, HACCP certification, ISO 9000 family, good manufacturing practices (GMP)/good hygiene practices (GHP), total quality management (TQM), good working practices (GWP), good lab practices (GLP), etc. Moreover, the role of some novel processing technologies is also focused on in this regard.
Quality Management System for HEIs
The development of quality management systems (QMS) in higher education institutions (HEIs) was driven on the one hand by the competitive pressure and, on the other hand, by growing concerns from stakeholders demanding assurance of institutional program quality and their graduates. Adopting QMS is currently mandatory, and quality accreditation, both institutional and program, becomes a plausible strategy to engage and continue offering programs by national and international bodies. This chapter offers an introductory understanding of the quality concept and quality management systems within higher education and sheds some light on the quality management system in Oman.
HOW ISO QUALITY MANAGEMENT STANDARDS CHANGE THE COMPANY LEVEL OF EXCELLENCE? STUDY CONDUCTED ON TWO COMPANIES OF POLISH DNA SEQUENCING INDUSTRY USING THE BUSINESS IMPROVEMENT MATRIX
This study examines the level of excellence of two companies, using Business Improvement Matrix (BIM), which is based on European Foundation of Quality Management’s Model of Excellence. It compares those organizations and shows how their quality management systems make an effect on BIM results. The researched companies are genXone S.A., which is in the process of implementing a quality management system compliant with ISO 13485: 2016, and Centrum Genetyki Medycznej GENESIS Sp. z o.o., certified with ISO 9001: 2015 since 2018. Both of them belong to sequencing industry in Poland, using new generation sequencing. This study shows that the company that has implemented its quality management system and has fully adopted its standards achieved a higher level of excellence than the company that just started the process of implementation of their quality management system.
Business Excellence Models and External Stakeholders Influencing the Late Adoption of Quality Management Systems in Zimbabwe Hotel Industry
The main objective of this paper was to determine business excellences and stakeholders influencing the late adoption of quality management systems in Zimbabwe hotel industry and suggest recommendation that encourages adoption of quality management systems. The study followed a multi case study approach, with 9 hotels from Harare chosen purposively to represent the hotel industry in Zimbabwe. In-depth interviews and focus group discussions were used to get data from hotel managers, key stakeholders and staff members. Directed content analysis was used to analyse data. The results revealed that hotels in Zimbabwe do not follow internationally recognised business excellence models. Five key external stakeholders – banks, Zimbabwe Tourism Authority, Tourism Business Council of Zimbabwe, Hospitality Association of Zimbabwe, tourists and Standard Association of Zimbabwe were identified to be influencing adoption of quality management systems in the hotel industry. The study recommended for the establishment of local business excellence models that are specific to hotels in Zimbabwe and for the government to avail accessible revolving bank loans for the hotels to invest towards quality management systems. The determination of the external stakeholders influencing late adoption of quality management systems in the hotel industry and use of business excellence models will help improve the adoption of QMSs under Zimbabwe's National Development Strategy 1 (NDS) to realise Vision 2030 "Towards a Prosperous and Empowered Upper Middle-Income Society".
ON THE FULL-FLEDGED MISSION STATEMENT OF THE UNIVERSITY SCIENTIFIC LIBRARY
The mission statement of an organization compiled in accordance with the definition of the term “mission” of the dictionary of the ISO 9000 quality management systems standard cannot be recognized as an effective management tool, since the ISO requirements for this document are too generic and vague. The objectives of this work were to clarify the functions of the mission statement of the university library as a management document, to develop a definition of the mission statement, consisting mainly of a list of its functions, and to develop a full-fledged mission statement of the Scientific Library of the Belarusian National Technical University based on the developed functional definition. Accordingly, the methods of work were the retrieval and interpretation of relevant scientific literature and the synthesis of data obtained from it for the development of new documents, viz. the definition of the mission statement and the mission statement of our library based on it. As a result, the intended functional definition of a university library mission statement as a full-fledged effective management document has been given and the mission statement of the Scientific Library of the Belarusian National Technical University has been crafted. The aspects of improving the efficiency of the library after the adoption of the new mission statement are briefly discussed.
Relevant Information for the Accountability of Private Institutions of Social Solidarity: Results from a Fieldwork
The Social Economy (SE) emerges as an interesting alternative to deal with social problems that often cannot be met by the services provided by the State. However, one of the concerns relates to the ability of these institutions to meet the demands of stakeholders concerning accountability. In this sense, the present work aimed to determine if the IPSS are prepared to meet the management requirements by increasing their accountability. For that purpose, we conducted qualitative research, with an exploratory focus, with 31 Portuguese Private Social Solidarity Institutions (IPSS). The interviews took place between June and July 2019, with those responsible for managing the entities. The interviews were guided on a semi-structured script based on the literature review. After Content Analysis, it was found that, in most of the institutions interviewed, the board does not use management tools, such as performance analysis, social impact assessment, strategic planning and quality management systems, even recognising the importance of using them. The fact is due to the lack of access or knowledge about its use. In addition, the majority of the IPSS interviewed showed concern about the transparency and ethics of managers. Current strategic management practices are remarkably targeted at companies in the for-profit sector and can compromise the principle of investments in human and social issues. Thus, the introduction of new activities can further reinforce the pressure felt by these institutions in carrying out operational activities.
Comparison of satisfaction of students in schools using quality management systems with students of mainstream schools
Export citation format, share document.
- Open access
- Published: 19 April 2024
A scoping review of continuous quality improvement in healthcare system: conceptualization, models and tools, barriers and facilitators, and impact
- Aklilu Endalamaw 1 , 2 ,
- Resham B Khatri 1 , 3 ,
- Tesfaye Setegn Mengistu 1 , 2 ,
- Daniel Erku 1 , 4 , 5 ,
- Eskinder Wolka 6 ,
- Anteneh Zewdie 6 &
- Yibeltal Assefa 1
BMC Health Services Research volume 24 , Article number: 487 ( 2024 ) Cite this article
11k Accesses
5 Citations
Metrics details
The growing adoption of continuous quality improvement (CQI) initiatives in healthcare has generated a surge in research interest to gain a deeper understanding of CQI. However, comprehensive evidence regarding the diverse facets of CQI in healthcare has been limited. Our review sought to comprehensively grasp the conceptualization and principles of CQI, explore existing models and tools, analyze barriers and facilitators, and investigate its overall impacts.
This qualitative scoping review was conducted using Arksey and O’Malley’s methodological framework. We searched articles in PubMed, Web of Science, Scopus, and EMBASE databases. In addition, we accessed articles from Google Scholar. We used mixed-method analysis, including qualitative content analysis and quantitative descriptive for quantitative findings to summarize findings and PRISMA extension for scoping reviews (PRISMA-ScR) framework to report the overall works.
A total of 87 articles, which covered 14 CQI models, were included in the review. While 19 tools were used for CQI models and initiatives, Plan-Do-Study/Check-Act cycle was the commonly employed model to understand the CQI implementation process. The main reported purposes of using CQI, as its positive impact, are to improve the structure of the health system (e.g., leadership, health workforce, health technology use, supplies, and costs), enhance healthcare delivery processes and outputs (e.g., care coordination and linkages, satisfaction, accessibility, continuity of care, safety, and efficiency), and improve treatment outcome (reduce morbidity and mortality). The implementation of CQI is not without challenges. There are cultural (i.e., resistance/reluctance to quality-focused culture and fear of blame or punishment), technical, structural (related to organizational structure, processes, and systems), and strategic (inadequate planning and inappropriate goals) related barriers that were commonly reported during the implementation of CQI.
Conclusions
Implementing CQI initiatives necessitates thoroughly comprehending key principles such as teamwork and timeline. To effectively address challenges, it’s crucial to identify obstacles and implement optimal interventions proactively. Healthcare professionals and leaders need to be mentally equipped and cognizant of the significant role CQI initiatives play in achieving purposes for quality of care.
Peer Review reports
Continuous quality improvement (CQI) initiative is a crucial initiative aimed at enhancing quality in the health system that has gradually been adopted in the healthcare industry. In the early 20th century, Shewhart laid the foundation for quality improvement by describing three essential steps for process improvement: specification, production, and inspection [ 1 , 2 ]. Then, Deming expanded Shewhart’s three-step model into ‘plan, do, study/check, and act’ (PDSA or PDCA) cycle, which was applied to management practices in Japan in the 1950s [ 3 ] and was gradually translated into the health system. In 1991, Kuperman applied a CQI approach to healthcare, comprising selecting a process to be improved, assembling a team of expert clinicians that understands the process and the outcomes, determining key steps in the process and expected outcomes, collecting data that measure the key process steps and outcomes, and providing data feedback to the practitioners [ 4 ]. These philosophies have served as the baseline for the foundation of principles for continuous improvement [ 5 ].
Continuous quality improvement fosters a culture of continuous learning, innovation, and improvement. It encourages proactive identification and resolution of problems, promotes employee engagement and empowerment, encourages trust and respect, and aims for better quality of care [ 6 , 7 ]. These characteristics drive the interaction of CQI with other quality improvement projects, such as quality assurance and total quality management [ 8 ]. Quality assurance primarily focuses on identifying deviations or errors through inspections, audits, and formal reviews, often settling for what is considered ‘good enough’, rather than pursuing the highest possible standards [ 9 , 10 ], while total quality management is implemented as the management philosophy and system to improve all aspects of an organization continuously [ 11 ].
Continuous quality improvement has been implemented to provide quality care. However, providing effective healthcare is a complicated and complex task in achieving the desired health outcomes and the overall well-being of individuals and populations. It necessitates tackling issues, including access, patient safety, medical advances, care coordination, patient-centered care, and quality monitoring [ 12 , 13 ], rooted long ago. It is assumed that the history of quality improvement in healthcare started in 1854 when Florence Nightingale introduced quality improvement documentation [ 14 ]. Over the passing decades, Donabedian introduced structure, processes, and outcomes as quality of care components in 1966 [ 15 ]. More comprehensively, the Institute of Medicine in the United States of America (USA) has identified effectiveness, efficiency, equity, patient-centredness, safety, and timeliness as the components of quality of care [ 16 ]. Moreover, quality of care has recently been considered an integral part of universal health coverage (UHC) [ 17 ], which requires initiatives to mobilise essential inputs [ 18 ].
While the overall objective of CQI in health system is to enhance the quality of care, it is important to note that the purposes and principles of CQI can vary across different contexts [ 19 , 20 ]. This variation has sparked growing research interest. For instance, a review of CQI approaches for capacity building addressed its role in health workforce development [ 21 ]. Another systematic review, based on random-controlled design studies, assessed the effectiveness of CQI using training as an intervention and the PDSA model [ 22 ]. As a research gap, the former review was not directly related to the comprehensive elements of quality of care, while the latter focused solely on the impact of training using the PDSA model, among other potential models. Additionally, a review conducted in 2015 aimed to identify barriers and facilitators of CQI in Canadian contexts [ 23 ]. However, all these reviews presented different perspectives and investigated distinct outcomes. This suggests that there is still much to explore in terms of comprehensively understanding the various aspects of CQI initiatives in healthcare.
As a result, we conducted a scoping review to address several aspects of CQI. Scoping reviews serve as a valuable tool for systematically mapping the existing literature on a specific topic. They are instrumental when dealing with heterogeneous or complex bodies of research. Scoping reviews provide a comprehensive overview by summarizing and disseminating findings across multiple studies, even when evidence varies significantly [ 24 ]. In our specific scoping review, we included various types of literature, including systematic reviews, to enhance our understanding of CQI.
This scoping review examined how CQI is conceptualized and measured and investigated models and tools for its application while identifying implementation challenges and facilitators. It also analyzed the purposes and impact of CQI on the health systems, providing valuable insights for enhancing healthcare quality.
Protocol registration and results reporting
Protocol registration for this scoping review was not conducted. Arksey and O’Malley’s methodological framework was utilized to conduct this scoping review [ 25 ]. The scoping review procedures start by defining the research questions, identifying relevant literature, selecting articles, extracting data, and summarizing the results. The review findings are reported using the PRISMA extension for a scoping review (PRISMA-ScR) [ 26 ]. McGowan and colleagues also advised researchers to report findings from scoping reviews using PRISMA-ScR [ 27 ].
Defining the research problems
This review aims to comprehensively explore the conceptualization, models, tools, barriers, facilitators, and impacts of CQI within the healthcare system worldwide. Specifically, we address the following research questions: (1) How has CQI been defined across various contexts? (2) What are the diverse approaches to implementing CQI in healthcare settings? (3) Which tools are commonly employed for CQI implementation ? (4) What barriers hinder and facilitators support successful CQI initiatives? and (5) What effects CQI initiatives have on the overall care quality?
Information source and search strategy
We conducted the search in PubMed, Web of Science, Scopus, and EMBASE databases, and the Google Scholar search engine. The search terms were selected based on three main distinct concepts. One group was CQI-related terms. The second group included terms related to the purpose for which CQI has been implemented, and the third group included processes and impact. These terms were selected based on the Donabedian framework of structure, process, and outcome [ 28 ]. Additionally, the detailed keywords were recruited from the primary health framework, which has described lists of dimensions under process, output, outcome, and health system goals of any intervention for health [ 29 ]. The detailed search strategy is presented in the Supplementary file 1 (Search strategy). The search for articles was initiated on August 12, 2023, and the last search was conducted on September 01, 2023.
Eligibility criteria and article selection
Based on the scoping review’s population, concept, and context frameworks [ 30 ], the population included any patients or clients. Additionally, the concepts explored in the review encompassed definitions, implementation, models, tools, barriers, facilitators, and impacts of CQI. Furthermore, the review considered contexts at any level of health systems. We included articles if they reported results of qualitative or quantitative empirical study, case studies, analytic or descriptive synthesis, any review, and other written documents, were published in peer-reviewed journals, and were designed to address at least one of the identified research questions or one of the identified implementation outcomes or their synonymous taxonomy as described in the search strategy. Based on additional contexts, we included articles published in English without geographic and time limitations. We excluded articles with abstracts only, conference abstracts, letters to editors, commentators, and corrections.
We exported all citations to EndNote x20 to remove duplicates and screen relevant articles. The article selection process includes automatic duplicate removal by using EndNote x20, unmatched title and abstract removal, citation and abstract-only materials removal, and full-text assessment. The article selection process was mainly conducted by the first author (AE) and reported to the team during the weekly meetings. The first author encountered papers that caused confusion regarding whether to include or exclude them and discussed them with the last author (YA). Then, decisions were ultimately made. Whenever disagreements happened, they were resolved by discussion and reconsideration of the review questions in relation to the written documents of the article. Further statistical analysis, such as calculating Kappa, was not performed to determine article inclusion or exclusion.
Data extraction and data items
We extracted first author, publication year, country, settings, health problem, the purpose of the study, study design, types of intervention if applicable, CQI approaches/steps if applicable, CQI tools and procedures if applicable, and main findings using a customized Microsoft Excel form.
Summarizing and reporting the results
The main findings were summarized and described based on the main themes, including concepts under conceptualizing, principles, teams, timelines, models, tools, barriers, facilitators, and impacts of CQI. Results-based convergent synthesis, achieved through mixed-method analysis, involved content analysis to identify the thematic presentation of findings. Additionally, a narrative description was used for quantitative findings, aligning them with the appropriate theme. The authors meticulously reviewed the primary findings from each included material and contextualized these findings concerning the main themes1. This approach provides a comprehensive understanding of complex interventions and health systems, acknowledging quantitative and qualitative evidence.
Search results
A total of 11,251 documents were identified from various databases: SCOPUS ( n = 4,339), PubMed ( n = 2,893), Web of Science ( n = 225), EMBASE ( n = 3,651), and Google Scholar ( n = 143). After removing duplicates ( n = 5,061), 6,190 articles were evaluated by title and abstract. Subsequently, 208 articles were assessed for full-text eligibility. Following the eligibility criteria, 121 articles were excluded, leaving 87 included in the current review (Fig. 1 ).
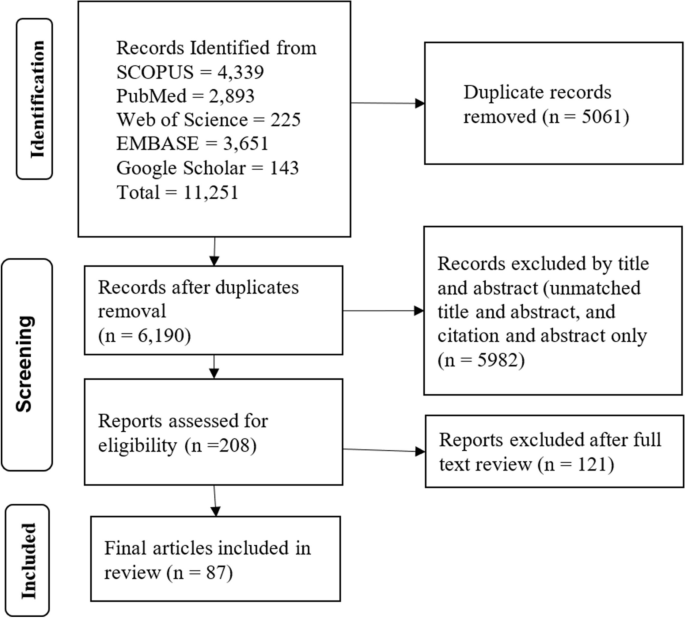
Article selection process
Operationalizing continuous quality improvement
Continuous Quality Improvement (CQI) is operationalized as a cyclic process that requires commitment to implementation, teamwork, time allocation, and celebrating successes and failures.
CQI is a cyclic ongoing process that is followed reflexive, analytical and iterative steps, including identifying gaps, generating data, developing and implementing action plans, evaluating performance, providing feedback to implementers and leaders, and proposing necessary adjustments [ 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 ].
CQI requires committing to the philosophy, involving continuous improvement [ 19 , 38 ], establishing a mission statement [ 37 ], and understanding quality definition [ 19 ].
CQI involves a wide range of patient-oriented measures and performance indicators, specifically satisfying internal and external customers, developing quality assurance, adopting common quality measures, and selecting process measures [ 8 , 19 , 35 , 36 , 37 , 39 , 40 ].
CQI requires celebrating success and failure without personalization, leading each team member to develop error-free attitudes [ 19 ]. Success and failure are related to underlying organizational processes and systems as causes of failure rather than blaming individuals [ 8 ] because CQI is process-focused based on collaborative, data-driven, responsive, rigorous and problem-solving statistical analysis [ 8 , 19 , 38 ]. Furthermore, a gap or failure opens another opportunity for establishing a data-driven learning organization [ 41 ].
CQI cannot be implemented without a CQI team [ 8 , 19 , 37 , 39 , 42 , 43 , 44 , 45 , 46 ]. A CQI team comprises individuals from various disciplines, often comprising a team leader, a subject matter expert (physician or other healthcare provider), a data analyst, a facilitator, frontline staff, and stakeholders [ 39 , 43 , 47 , 48 , 49 ]. It is also important to note that inviting stakeholders or partners as part of the CQI support intervention is crucial [ 19 , 38 , 48 ].
The timeline is another distinct feature of CQI because the results of CQI vary based on the implementation duration of each cycle [ 35 ]. There is no specific time limit for CQI implementation, although there is a general consensus that a cycle of CQI should be relatively short [ 35 ]. For instance, a CQI implementation took 2 months [ 42 ], 4 months [ 50 ], 9 months [ 51 , 52 ], 12 months [ 53 , 54 , 55 ], and one year and 5 months [ 49 ] duration to achieve the desired positive outcome, while bi-weekly [ 47 ] and monthly data reviews and analyses [ 44 , 48 , 56 ], and activities over 3 months [ 57 ] have also resulted in a positive outcome.
Continuous quality improvement models and tools
There have been several models are utilized. The Plan-Do-Study/Check-Act cycle is a stepwise process involving project initiation, situation analysis, root cause identification, solution generation and selection, implementation, result evaluation, standardization, and future planning [ 7 , 36 , 37 , 45 , 47 , 48 , 49 , 50 , 51 , 53 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 ]. The FOCUS-PDCA cycle enhances the PDCA process by adding steps to find and improve a process (F), organize a knowledgeable team (O), clarify the process (C), understand variations (U), and select improvements (S) [ 55 , 71 , 72 , 73 ]. The FADE cycle involves identifying a problem (Focus), understanding it through data analysis (Analyze), devising solutions (Develop), and implementing the plan (Execute) [ 74 ]. The Logic Framework involves brainstorming to identify improvement areas, conducting root cause analysis to develop a problem tree, logically reasoning to create an objective tree, formulating the framework, and executing improvement projects [ 75 ]. Breakthrough series approach requires CQI teams to meet in quarterly collaborative learning sessions, share learning experiences, and continue discussion by telephone and cross-site visits to strengthen learning and idea exchange [ 47 ]. Another CQI model is the Lean approach, which has been conducted with Kaizen principles [ 52 ], 5 S principles, and the Six Sigma model. The 5 S (Sort, Set/Straighten, Shine, Standardize, Sustain) systematically organises and improves the workplace, focusing on sorting, setting order, shining, standardizing, and sustaining the improvement [ 54 , 76 ]. Kaizen principles guide CQI by advocating for continuous improvement, valuing all ideas, solving problems, focusing on practical, low-cost improvements, using data to drive change, acknowledging process defects, reducing variability and waste, recognizing every interaction as a customer-supplier relationship, empowering workers, responding to all ideas, and maintaining a disciplined workplace [ 77 ]. Lean Six Sigma, a CQI model, applies the DMAIC methodology, which involves defining (D) and measuring the problem (M), analyzing root causes (A), improving by finding solutions (I), and controlling by assessing process stability (C) [ 78 , 79 ]. The 5 C-cyclic model (consultation, collection, consideration, collaboration, and celebration), the first CQI framework for volunteer dental services in Aboriginal communities, ensures quality care based on community needs [ 80 ]. One study used meetings involving activities such as reviewing objectives, assigning roles, discussing the agenda, completing tasks, retaining key outputs, planning future steps, and evaluating the meeting’s effectiveness [ 81 ].
Various tools are involved in the implementation or evaluation of CQI initiatives: checklists [ 53 , 82 ], flowcharts [ 81 , 82 , 83 ], cause-and-effect diagrams (fishbone or Ishikawa diagrams) [ 60 , 62 , 79 , 81 , 82 ], fuzzy Pareto diagram [ 82 ], process maps [ 60 ], time series charts [ 48 ], why-why analysis [ 79 ], affinity diagrams and multivoting [ 81 ], and run chart [ 47 , 48 , 51 , 60 , 84 ], and others mentioned in the table (Table 1 ).
Barriers and facilitators of continuous quality improvement implementation
Implementing CQI initiatives is determined by various barriers and facilitators, which can be thematized into four dimensions. These dimensions are cultural, technical, structural, and strategic dimensions.
Continuous quality improvement initiatives face various cultural, strategic, technical, and structural barriers. Cultural dimension barriers involve resistance to change (e.g., not accepting online technology), lack of quality-focused culture, staff reporting apprehensiveness, and fear of blame or punishment [ 36 , 41 , 85 , 86 ]. The technical dimension barriers of CQI can include various factors that hinder the effective implementation and execution of CQI processes [ 36 , 86 , 87 , 88 , 89 ]. Structural dimension barriers of CQI arise from the organization structure, process, and systems that can impede the effective implementation and sustainability of CQI [ 36 , 85 , 86 , 87 , 88 ]. Strategic dimension barriers are, for example, the inability to select proper CQI goals and failure to integrate CQI into organizational planning and goals [ 36 , 85 , 86 , 87 , 88 , 90 ].
Facilitators are also grouped to cultural, structural, technical, and strategic dimensions to provide solutions to CQI barriers. Cultural challenges were addressed by developing a group culture to CQI and other rewards [ 39 , 41 , 80 , 85 , 86 , 87 , 90 , 91 , 92 ]. Technical facilitators are pivotal to improving technical barriers [ 39 , 42 , 53 , 69 , 86 , 90 , 91 ]. Structural-related facilitators are related to improving communication, infrastructure, and systems [ 86 , 92 , 93 ]. Strategic dimension facilitators include strengthening leadership and improving decision-making skills [ 43 , 53 , 67 , 86 , 87 , 92 , 94 , 95 ] (Table 2 ).
Impact of continuous quality improvement
Continuous quality improvement initiatives can significantly impact the quality of healthcare in a wide range of health areas, focusing on improving structure, the health service delivery process and improving client wellbeing and reducing mortality.
Structure components
These are health leadership, financing, workforce, technology, and equipment and supplies. CQI has improved planning, monitoring and evaluation [ 48 , 53 ], and leadership and planning [ 48 ], indicating improvement in leadership perspectives. Implementing CQI in primary health care (PHC) settings has shown potential for maintaining or reducing operation costs [ 67 ]. Findings from another study indicate that the costs associated with implementing CQI interventions per facility ranged from approximately $2,000 to $10,500 per year, with an average cost of approximately $10 to $60 per admitted client [ 57 ]. However, based on model predictions, the average cost savings after implementing CQI were estimated to be $5430 [ 31 ]. CQI can also be applied to health workforce development [ 32 ]. CQI in the institutional system improved medical education [ 66 , 96 , 97 ], human resources management [ 53 ], motivated staffs [ 76 ], and increased staff health awareness [ 69 ], while concerns raised about CQI impartiality, independence, and public accountability [ 96 ]. Regarding health technology, CQI also improved registration and documentation [ 48 , 53 , 98 ]. Furthermore, the CQI initiatives increased cleanliness [ 54 ] and improved logistics, supplies, and equipment [ 48 , 53 , 68 ].
Process and output components
The process component focuses on the activities and actions involved in delivering healthcare services.
Service delivery
CQI interventions improved service delivery [ 53 , 56 , 99 ], particularly a significant 18% increase in the overall quality of service performance [ 48 ], improved patient counselling, adherence to appropriate procedures, and infection prevention [ 48 , 68 ], and optimised workflow [ 52 ].
Coordination and collaboration
CQI initiatives improved coordination and collaboration through collecting and analysing data, onsite technical support, training, supportive supervision [ 53 ] and facilitating linkages between work processes and a quality control group [ 65 ].
Patient satisfaction
The CQI initiatives increased patient satisfaction and improved quality of life by optimizing care quality management, improving the quality of clinical nursing, reducing nursing defects and enhancing the wellbeing of clients [ 54 , 76 , 100 ], although CQI was not associated with changes in adolescent and young adults’ satisfaction [ 51 ].
CQI initiatives reduced medication error reports from 16 to 6 [ 101 ], and it significantly reduced the administration of inappropriate prophylactic antibiotics [ 44 ], decreased errors in inpatient care [ 52 ], decreased the overall episiotomy rate from 44.5 to 33.3% [ 83 ], reduced the overall incidence of unplanned endotracheal extubation [ 102 ], improving appropriate use of computed tomography angiography [ 103 ], and appropriate diagnosis and treatment selection [ 47 ].
Continuity of care
CQI initiatives effectively improve continuity of care by improving client and physician interaction. For instance, provider continuity levels showed a 64% increase [ 55 ]. Modifying electronic medical record templates, scheduling, staff and parental education, standardization of work processes, and birth to 1-year age-specific incentives in post-natal follow-up care increased continuity of care to 74% in 2018 compared to baseline 13% in 2012 [ 84 ].
The CQI initiative yielded enhanced efficiency in the cardiac catheterization laboratory, as evidenced by improved punctuality in procedure starts and increased efficiency in manual sheath-pulls inside [ 78 ].
Accessibility
CQI initiatives were effective in improving accessibility in terms of increasing service coverage and utilization rate. For instance, screening for cigarettes, nutrition counselling, folate prescription, maternal care, immunization coverage [ 53 , 81 , 104 , 105 ], reducing the percentage of non-attending patients to surgery to 0.9% from the baseline 3.9% [ 43 ], increasing Chlamydia screening rates from 29 to 60% [ 45 ], increasing HIV care continuum coverage [ 51 , 59 , 60 ], increasing in the uptake of postpartum long-acting reversible contraceptive use from 6.9% at the baseline to 25.4% [ 42 ], increasing post-caesarean section prophylaxis from 36 to 89% [ 62 ], a 31% increase of kangaroo care practice [ 50 ], and increased follow-up [ 65 ]. Similarly, the QI intervention increased the quality of antenatal care by 29.3%, correct partograph use by 51.7%, and correct active third-stage labour management, a 19.6% improvement from the baseline, but not significantly associated with improvement in contraceptive service uptake [ 61 ].
Timely access
CQI interventions improved the time care provision [ 52 ], and reduced waiting time [ 62 , 74 , 76 , 106 ]. For instance, the discharge process waiting time in the emergency department decreased from 76 min to 22 min [ 79 ]. It also reduced mean postprocedural length of stay from 2.8 days to 2.0 days [ 31 ].
Acceptability
Acceptability of CQI by healthcare providers was satisfactory. For instance, 88% of the faculty, 64% of the residents, and 82% of the staff believed CQI to be useful in the healthcare clinic [ 107 ].
Outcome components
Morbidity and mortality.
CQI efforts have demonstrated better management outcomes among diabetic patients [ 40 ], patients with oral mucositis [ 71 ], and anaemic patients [ 72 ]. It has also reduced infection rate in post-caesarean Sect. [ 62 ], reduced post-peritoneal dialysis peritonitis [ 49 , 108 ], and prevented pressure ulcers [ 70 ]. It is explained by peritonitis incidence from once every 40.1 patient months at baseline to once every 70.8 patient months after CQI [ 49 ] and a 63% reduction in pressure ulcer prevalence within 2 years from 2008 to 2010 [ 70 ]. Furthermore, CQI initiatives significantly reduced in-hospital deaths [ 31 ] and increased patient survival rates [ 108 ]. Figure 2 displays the overall process of the CQI implementations.
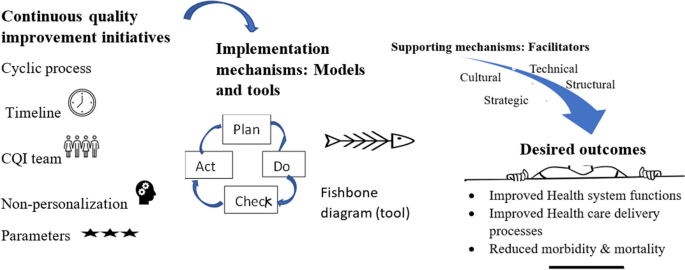
The overall mechanisms of continuous quality improvement implementation
In this review, we examined the fundamental concepts and principles underlying CQI, the factors that either hinder or assist in its successful application and implementation, and the purpose of CQI in enhancing quality of care across various health issues.
Our findings have brought attention to the application and implementation of CQI, emphasizing its underlying concepts and principles, as evident in the existing literature [ 31 , 32 , 33 , 34 , 35 , 36 , 39 , 40 , 43 , 45 , 46 ]. Continuous quality improvement has shared with the principles of continuous improvement, such as a customer-driven focus, effective leadership, active participation of individuals, a process-oriented approach, systematic implementation, emphasis on design improvement and prevention, evidence-based decision-making, and fostering partnership [ 5 ]. Moreover, Deming’s 14 principles laid the foundation for CQI principles [ 109 ]. These principles have been adapted and put into practice in various ways: ten [ 19 ] and five [ 38 ] principles in hospitals, five principles for capacity building [ 38 ], and two principles for medication error prevention [ 41 ]. As a principle, the application of CQI can be process-focused [ 8 , 19 ] or impact-focused [ 38 ]. Impact-focused CQI focuses on achieving specific outcomes or impacts, whereas process-focused CQI prioritizes and improves the underlying processes and systems. These principles complement each other and can be utilized based on the objectives of quality improvement initiatives in healthcare settings. Overall, CQI is an ongoing educational process that requires top management’s involvement, demands coordination across departments, encourages the incorporation of views beyond clinical area, and provides non-judgemental evidence based on objective data [ 110 ].
The current review recognized that it was not easy to implement CQI. It requires reasonable utilization of various models and tools. The application of each tool can be varied based on the studied health problem and the purpose of CQI initiative [ 111 ], varied in context, content, structure, and usability [ 112 ]. Additionally, overcoming the cultural, technical, structural, and strategic-related barriers. These barriers have emerged from clinical staff, managers, and health systems perspectives. Of the cultural obstacles, staff non-involvement, resistance to change, and reluctance to report error were staff-related. In contrast, others, such as the absence of celebration for success and hierarchical and rational culture, may require staff and manager involvement. Staff members may exhibit reluctance in reporting errors due to various cultural factors, including lack of trust, hierarchical structures, fear of retribution, and a blame-oriented culture. These challenges pose obstacles to implementing standardized CQI practices, as observed, for instance, in community pharmacy settings [ 85 ]. The hierarchical culture, characterized by clearly defined levels of power, authority, and decision-making, posed challenges to implementing CQI initiatives in public health [ 41 , 86 ]. Although rational culture, a type of organizational culture, emphasizes logical thinking and rational decision-making, it can also create challenges for CQI implementation [ 41 , 86 ] because hierarchical and rational cultures, which emphasize bureaucratic norms and narrow definitions of achievement, were found to act as barriers to the implementation of CQI [ 86 ]. These could be solved by developing a shared mindset and collective commitment, establishing a shared purpose, developing group norms, and cultivating psychological preparedness among staff, managers, and clients to implement and sustain CQI initiatives. Furthermore, reversing cultural-related barriers necessitates cultural-related solutions: development of a culture and group culture to CQI [ 41 , 86 ], positive comprehensive perception [ 91 ], commitment [ 85 ], involving patients, families, leaders, and staff [ 39 , 92 ], collaborating for a common goal [ 80 , 86 ], effective teamwork [ 86 , 87 ], and rewarding and celebrating successes [ 80 , 90 ].
The technical dimension barriers of CQI can include inadequate capitalization of a project and insufficient support for CQI facilitators and data entry managers [ 36 ], immature electronic medical records or poor information systems [ 36 , 86 ], and the lack of training and skills [ 86 , 87 , 88 ]. These challenges may cause the CQI team to rely on outdated information and technologies. The presence of barriers on the technical dimension may challenge the solid foundation of CQI expertise among staff, the ability to recognize opportunities for improvement, a comprehensive understanding of how services are produced and delivered, and routine use of expertise in daily work. Addressing these technical barriers requires knowledge creation activities (training, seminar, and education) [ 39 , 42 , 53 , 69 , 86 , 90 , 91 ], availability of quality data [ 86 ], reliable information [ 92 ], and a manual-online hybrid reporting system [ 85 ].
Structural dimension barriers of CQI include inadequate communication channels and lack of standardized process, specifically weak physician-to-physician synergies [ 36 ], lack of mechanisms for disseminating knowledge and limited use of communication mechanisms [ 86 ]. Lack of communication mechanism endangers sharing ideas and feedback among CQI teams, leading to misunderstandings, limited participation and misinterpretations, and a lack of learning [ 113 ]. Knowledge translation facilitates the co-production of research, subsequent diffusion of knowledge, and the developing stakeholder’s capacity and skills [ 114 ]. Thus, the absence of a knowledge translation mechanism may cause missed opportunities for learning, inefficient problem-solving, and limited creativity. To overcome these challenges, organizations should establish effective communication and information systems [ 86 , 93 ] and learning systems [ 92 ]. Though CQI and knowledge translation have interacted with each other, it is essential to recognize that they are distinct. CQI focuses on process improvement within health care systems, aiming to optimize existing processes, reduce errors, and enhance efficiency.
In contrast, knowledge translation bridges the gap between research evidence and clinical practice, translating research findings into actionable knowledge for practitioners. While both CQI and knowledge translation aim to enhance health care quality and patient outcomes, they employ different strategies: CQI utilizes tools like Plan-Do-Study-Act cycles and statistical process control, while knowledge translation involves knowledge synthesis and dissemination. Additionally, knowledge translation can also serve as a strategy to enhance CQI. Both concepts share the same principle: continuous improvement is essential for both. Therefore, effective strategies on the structural dimension may build efficient and effective steering councils, information systems, and structures to diffuse learning throughout the organization.
Strategic factors, such as goals, planning, funds, and resources, determine the overall purpose of CQI initiatives. Specific barriers were improper goals and poor planning [ 36 , 86 , 88 ], fragmentation of quality assurance policies [ 87 ], inadequate reinforcement to staff [ 36 , 90 ], time constraints [ 85 , 86 ], resource inadequacy [ 86 ], and work overload [ 86 ]. These barriers can be addressed through strengthening leadership [ 86 , 87 ], CQI-based mentoring [ 94 ], periodic monitoring, supportive supervision and coaching [ 43 , 53 , 87 , 92 , 95 ], participation, empowerment, and accountability [ 67 ], involving all stakeholders in decision-making [ 86 , 87 ], a provider-payer partnership [ 64 ], and compensating staff for after-hours meetings on CQI [ 85 ]. The strategic dimension, characterized by a strategic plan and integrated CQI efforts, is devoted to processes that are central to achieving strategic priorities. Roles and responsibilities are defined in terms of integrated strategic and quality-related goals [ 115 ].
The utmost goal of CQI has been to improve the quality of care, which is usually revealed by structure, process, and outcome. After resolving challenges and effectively using tools and running models, the goal of CQI reflects the ultimate reason and purpose of its implementation. First, effectively implemented CQI initiatives can improve leadership, health financing, health workforce development, health information technology, and availability of supplies as the building blocks of a health system [ 31 , 48 , 53 , 68 , 98 ]. Second, effectively implemented CQI initiatives improved care delivery process (counselling, adherence with standards, coordination, collaboration, and linkages) [ 48 , 53 , 65 , 68 ]. Third, the CQI can improve outputs of healthcare delivery, such as satisfaction, accessibility (timely access, utilization), continuity of care, safety, efficiency, and acceptability [ 52 , 54 , 55 , 76 , 78 ]. Finally, the effectiveness of the CQI initiatives has been tested in enhancing responses related to key aspects of the HIV response, maternal and child health, non-communicable disease control, and others (e.g., surgery and peritonitis). However, it is worth noting that CQI initiative has not always been effective. For instance, CQI using a two- to nine-times audit cycle model through systems assessment tools did not bring significant change to increase syphilis testing performance [ 116 ]. This study was conducted within the context of Aboriginal and Torres Strait Islander people’s primary health care settings. Notably, ‘the clinics may not have consistently prioritized syphilis testing performance in their improvement strategies, as facilitated by the CQI program’ [ 116 ]. Additionally, by applying CQI-based mentoring, uptake of facility-based interventions was not significantly improved, though it was effective in increasing community health worker visits during pregnancy and the postnatal period, knowledge about maternal and child health and exclusive breastfeeding practice, and HIV disclosure status [ 117 ]. The study conducted in South Africa revealed no significant association between the coverage of facility-based interventions and Continuous Quality Improvement (CQI) implementation. This lack of association was attributed to the already high antenatal and postnatal attendance rates in both control and intervention groups at baseline, leaving little room for improvement. Additionally, the coverage of HIV interventions remained consistently high throughout the study period [ 117 ].
Regarding health care and policy implications, CQI has played a vital role in advancing PHC and fostering the realization of UHC goals worldwide. The indicators found in Donabedian’s framework that are positively influenced by CQI efforts are comparable to those included in the PHC performance initiative’s conceptual framework [ 29 , 118 , 119 ]. It is clearly explained that PHC serves as the roadmap to realizing the vision of UHC [ 120 , 121 ]. Given these circumstances, implementing CQI can contribute to the achievement of PHC principles and the objectives of UHC. For instance, by implementing CQI methods, countries have enhanced the accessibility, affordability, and quality of PHC services, leading to better health outcomes for their populations. CQI has facilitated identifying and resolving healthcare gaps and inefficiencies, enabling countries to optimize resource allocation and deliver more effective and patient-centered care. However, it is crucial to recognize that the successful implementation of Continuous Quality Improvement (CQI) necessitates optimizing the duration of each cycle, understanding challenges and barriers that extend beyond the health system and settings, and acknowledging that its effectiveness may be compromised if these challenges are not adequately addressed.
Despite abundant literature, there are still gaps regarding the relationship between CQI and other dimensions within the healthcare system. No studies have examined the impact of CQI initiatives on catastrophic health expenditure, effective service coverage, patient-centredness, comprehensiveness, equity, health security, and responsiveness.
Limitations
In conducting this review, it has some limitations to consider. Firstly, only articles published in English were included, which may introduce the exclusion of relevant non-English articles. Additionally, as this review follows a scoping methodology, the focus is on synthesising available evidence rather than critically evaluating or scoring the quality of the included articles.
Continuous quality improvement is investigated as a continuous and ongoing intervention, where the implementation time can vary across different cycles. The CQI team and implementation timelines were critical elements of CQI in different models. Among the commonly used approaches, the PDSA or PDCA is frequently employed. In most CQI models, a wide range of tools, nineteen tools, are commonly utilized to support the improvement process. Cultural, technical, structural, and strategic barriers and facilitators are significant in implementing CQI initiatives. Implementing the CQI initiative aims to improve health system blocks, enhance health service delivery process and output, and ultimately prevent morbidity and reduce mortality. For future researchers, considering that CQI is context-dependent approach, conducting scale-up implementation research about catastrophic health expenditure, effective service coverage, patient-centredness, comprehensiveness, equity, health security, and responsiveness across various settings and health issues would be valuable.
Availability of data and materials
The data used and/or analyzed during the current study are available in this manuscript and/or the supplementary file.
Shewhart WA, Deming WE. Memoriam: Walter A. Shewhart, 1891–1967. Am Stat. 1967;21(2):39–40.
Article Google Scholar
Shewhart WA. Statistical method from the viewpoint of quality control. New York: Dover; 1986. ISBN 978-0486652320. OCLC 13822053. Reprint. Originally published: Washington, DC: Graduate School of the Department of Agriculture, 1939.
Moen R, editor Foundation and History of the PDSA Cycle. Asian network for quality conference Tokyo. https://www.deming.org/sites/default/files/pdf/2015/PDSA_History_Ron_MoenPdf . 2009.
Kuperman G, James B, Jacobsen J, Gardner RM. Continuous quality improvement applied to medical care: experiences at LDS hospital. Med Decis Making. 1991;11(4suppl):S60–65.
Article CAS PubMed Google Scholar
Singh J, Singh H. Continuous improvement philosophy–literature review and directions. Benchmarking: An International Journal. 2015;22(1):75–119.
Goldstone J. Presidential address: Sony, Porsche, and vascular surgery in the 21st century. J Vasc Surg. 1997;25(2):201–10.
Radawski D. Continuous quality improvement: origins, concepts, problems, and applications. J Physician Assistant Educ. 1999;10(1):12–6.
Shortell SM, O’Brien JL, Carman JM, Foster RW, Hughes E, Boerstler H, et al. Assessing the impact of continuous quality improvement/total quality management: concept versus implementation. Health Serv Res. 1995;30(2):377.
CAS PubMed PubMed Central Google Scholar
Lohr K. Quality of health care: an introduction to critical definitions, concepts, principles, and practicalities. Striving for quality in health care. 1991.
Berwick DM. The clinical process and the quality process. Qual Manage Healthc. 1992;1(1):1–8.
Article CAS Google Scholar
Gift B. On the road to TQM. Food Manage. 1992;27(4):88–9.
CAS PubMed Google Scholar
Greiner A, Knebel E. The core competencies needed for health care professionals. health professions education: A bridge to quality. 2003:45–73.
McCalman J, Bailie R, Bainbridge R, McPhail-Bell K, Percival N, Askew D et al. Continuous quality improvement and comprehensive primary health care: a systems framework to improve service quality and health outcomes. Front Public Health. 2018:6 (76):1–6.
Sheingold BH, Hahn JA. The history of healthcare quality: the first 100 years 1860–1960. Int J Afr Nurs Sci. 2014;1:18–22.
Google Scholar
Donabedian A. Evaluating the quality of medical care. Milbank Q. 1966;44(3):166–206.
Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington (DC): National Academies Press (US). 2001. 2, Improving the 21st-century Health Care System. Available from: https://www.ncbi.nlm.nih.gov/books/NBK222265/ .
Rubinstein A, Barani M, Lopez AS. Quality first for effective universal health coverage in low-income and middle-income countries. Lancet Global Health. 2018;6(11):e1142–1143.
Article PubMed Google Scholar
Agency for Healthcare Reserach and Quality. Quality Improvement and monitoring at your fingertips USA,: Agency for Healthcare Reserach and Quality. 2022. Available from: https://qualityindicators.ahrq.gov/ .
Anderson CA, Cassidy B, Rivenburgh P. Implementing continuous quality improvement (CQI) in hospitals: lessons learned from the International Quality Study. Qual Assur Health Care. 1991;3(3):141–6.
Gardner K, Mazza D. Quality in general practice - definitions and frameworks. Aust Fam Physician. 2012;41(3):151–4.
PubMed Google Scholar
Loper AC, Jensen TM, Farley AB, Morgan JD, Metz AJ. A systematic review of approaches for continuous quality improvement capacity-building. J Public Health Manage Pract. 2022;28(2):E354.
Hill JE, Stephani A-M, Sapple P, Clegg AJ. The effectiveness of continuous quality improvement for developing professional practice and improving health care outcomes: a systematic review. Implement Sci. 2020;15(1):1–14.
Candas B, Jobin G, Dubé C, Tousignant M, Abdeljelil AB, Grenier S, et al. Barriers and facilitators to implementing continuous quality improvement programs in colonoscopy services: a mixed methods systematic review. Endoscopy Int Open. 2016;4(02):E118–133.
Peters MD, Marnie C, Colquhoun H, Garritty CM, Hempel S, Horsley T, et al. Scoping reviews: reinforcing and advancing the methodology and application. Syst Reviews. 2021;10(1):1–6.
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32.
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73.
McGowan J, Straus S, Moher D, Langlois EV, O’Brien KK, Horsley T, et al. Reporting scoping reviews—PRISMA ScR extension. J Clin Epidemiol. 2020;123:177–9.
Donabedian A. Explorations in quality assessment and monitoring: the definition of quality and approaches to its assessment. Health Administration Press, Ann Arbor. 1980;1.
World Health Organization. Operational framework for primary health care: transforming vision into action. Geneva: World Health Organization and the United Nations Children’s Fund (UNICEF); 2020 [updated 14 December 2020; cited 2023 Nov Oct 17]. Available from: https://www.who.int/publications/i/item/9789240017832 .
The Joanna Briggs Institute. The Joanna Briggs Institute Reviewers’ Manual :2014 edition. Australia: The Joanna Briggs Institute. 2014:88–91.
Rihal CS, Kamath CC, Holmes DR Jr, Reller MK, Anderson SS, McMurtry EK, et al. Economic and clinical outcomes of a physician-led continuous quality improvement intervention in the delivery of percutaneous coronary intervention. Am J Manag Care. 2006;12(8):445–52.
Ade-Oshifogun JB, Dufelmeier T. Prevention and Management of Do not return notices: a quality improvement process for Supplemental staffing nursing agencies. Nurs Forum. 2012;47(2):106–12.
Rubenstein L, Khodyakov D, Hempel S, Danz M, Salem-Schatz S, Foy R, et al. How can we recognize continuous quality improvement? Int J Qual Health Care. 2014;26(1):6–15.
O’Neill SM, Hempel S, Lim YW, Danz MS, Foy R, Suttorp MJ, et al. Identifying continuous quality improvement publications: what makes an improvement intervention ‘CQI’? BMJ Qual Saf. 2011;20(12):1011–9.
Article PubMed PubMed Central Google Scholar
Sibthorpe B, Gardner K, McAullay D. Furthering the quality agenda in Aboriginal community controlled health services: understanding the relationship between accreditation, continuous quality improvement and national key performance indicator reporting. Aust J Prim Health. 2016;22(4):270–5.
Bennett CL, Crane JM. Quality improvement efforts in oncology: are we ready to begin? Cancer Invest. 2001;19(1):86–95.
VanValkenburgh DA. Implementing continuous quality improvement at the facility level. Adv Ren Replace Ther. 2001;8(2):104–13.
Loper AC, Jensen TM, Farley AB, Morgan JD, Metz AJ. A systematic review of approaches for continuous quality improvement capacity-building. J Public Health Manage Practice. 2022;28(2):E354–361.
Ryan M. Achieving and sustaining quality in healthcare. Front Health Serv Manag. 2004;20(3):3–11.
Nicolucci A, Allotta G, Allegra G, Cordaro G, D’Agati F, Di Benedetto A, et al. Five-year impact of a continuous quality improvement effort implemented by a network of diabetes outpatient clinics. Diabetes Care. 2008;31(1):57–62.
Wakefield BJ, Blegen MA, Uden-Holman T, Vaughn T, Chrischilles E, Wakefield DS. Organizational culture, continuous quality improvement, and medication administration error reporting. Am J Med Qual. 2001;16(4):128–34.
Sori DA, Debelew GT, Degefa LS, Asefa Z. Continuous quality improvement strategy for increasing immediate postpartum long-acting reversible contraceptive use at Jimma University Medical Center, Jimma, Ethiopia. BMJ Open Qual. 2023;12(1):e002051.
Roche B, Robin C, Deleaval PJ, Marti MC. Continuous quality improvement in ambulatory surgery: the non-attending patient. Ambul Surg. 1998;6(2):97–100.
O’Connor JB, Sondhi SS, Mullen KD, McCullough AJ. A continuous quality improvement initiative reduces inappropriate prescribing of prophylactic antibiotics for endoscopic procedures. Am J Gastroenterol. 1999;94(8):2115–21.
Ursu A, Greenberg G, McKee M. Continuous quality improvement methodology: a case study on multidisciplinary collaboration to improve chlamydia screening. Fam Med Community Health. 2019;7(2):e000085.
Quick B, Nordstrom S, Johnson K. Using continuous quality improvement to implement evidence-based medicine. Lippincotts Case Manag. 2006;11(6):305–15 ( quiz 16 – 7 ).
Oyeledun B, Phillips A, Oronsaye F, Alo OD, Shaffer N, Osibo B, et al. The effect of a continuous quality improvement intervention on retention-in-care at 6 months postpartum in a PMTCT Program in Northern Nigeria: results of a cluster randomized controlled study. J Acquir Immune Defic Syndr. 2017;75(Suppl 2):S156–164.
Nyengerai T, Phohole M, Iqaba N, Kinge CW, Gori E, Moyo K, et al. Quality of service and continuous quality improvement in voluntary medical male circumcision programme across four provinces in South Africa: longitudinal and cross-sectional programme data. PLoS ONE. 2021;16(8):e0254850.
Article CAS PubMed PubMed Central Google Scholar
Wang J, Zhang H, Liu J, Zhang K, Yi B, Liu Y, et al. Implementation of a continuous quality improvement program reduces the occurrence of peritonitis in PD. Ren Fail. 2014;36(7):1029–32.
Stikes R, Barbier D. Applying the plan-do-study-act model to increase the use of kangaroo care. J Nurs Manag. 2013;21(1):70–8.
Wagner AD, Mugo C, Bluemer-Miroite S, Mutiti PM, Wamalwa DC, Bukusi D, et al. Continuous quality improvement intervention for adolescent and young adult HIV testing services in Kenya improves HIV knowledge. AIDS. 2017;31(Suppl 3):S243–252.
Le RD, Melanson SE, Santos KS, Paredes JD, Baum JM, Goonan EM, et al. Using lean principles to optimise inpatient phlebotomy services. J Clin Pathol. 2014;67(8):724–30.
Manyazewal T, Mekonnen A, Demelew T, Mengestu S, Abdu Y, Mammo D, et al. Improving immunization capacity in Ethiopia through continuous quality improvement interventions: a prospective quasi-experimental study. Infect Dis Poverty. 2018;7:7.
Kamiya Y, Ishijma H, Hagiwara A, Takahashi S, Ngonyani HAM, Samky E. Evaluating the impact of continuous quality improvement methods at hospitals in Tanzania: a cluster-randomized trial. Int J Qual Health Care. 2017;29(1):32–9.
Kibbe DC, Bentz E, McLaughlin CP. Continuous quality improvement for continuity of care. J Fam Pract. 1993;36(3):304–8.
Adrawa N, Ongiro S, Lotee K, Seret J, Adeke M, Izudi J. Use of a context-specific package to increase sputum smear monitoring among people with pulmonary tuberculosis in Uganda: a quality improvement study. BMJ Open Qual. 2023;12(3):1–6.
Hunt P, Hunter SB, Levan D. Continuous quality improvement in substance abuse treatment facilities: how much does it cost? J Subst Abuse Treat. 2017;77:133–40.
Azadeh A, Ameli M, Alisoltani N, Motevali Haghighi S. A unique fuzzy multi-control approach for continuous quality improvement in a radio therapy department. Qual Quantity. 2016;50(6):2469–93.
Memiah P, Tlale J, Shimabale M, Nzyoka S, Komba P, Sebeza J, et al. Continuous quality improvement (CQI) institutionalization to reach 95:95:95 HIV targets: a multicountry experience from the Global South. BMC Health Serv Res. 2021;21(1):711.
Yapa HM, De Neve JW, Chetty T, Herbst C, Post FA, Jiamsakul A, et al. The impact of continuous quality improvement on coverage of antenatal HIV care tests in rural South Africa: results of a stepped-wedge cluster-randomised controlled implementation trial. PLoS Med. 2020;17(10):e1003150.
Dadi TL, Abebo TA, Yeshitla A, Abera Y, Tadesse D, Tsegaye S, et al. Impact of quality improvement interventions on facility readiness, quality and uptake of maternal and child health services in developing regions of Ethiopia: a secondary analysis of programme data. BMJ Open Qual. 2023;12(4):e002140.
Weinberg M, Fuentes JM, Ruiz AI, Lozano FW, Angel E, Gaitan H, et al. Reducing infections among women undergoing cesarean section in Colombia by means of continuous quality improvement methods. Arch Intern Med. 2001;161(19):2357–65.
Andreoni V, Bilak Y, Bukumira M, Halfer D, Lynch-Stapleton P, Perez C. Project management: putting continuous quality improvement theory into practice. J Nurs Care Qual. 1995;9(3):29–37.
Balfour ME, Zinn TE, Cason K, Fox J, Morales M, Berdeja C, et al. Provider-payer partnerships as an engine for continuous quality improvement. Psychiatric Serv. 2018;69(6):623–5.
Agurto I, Sandoval J, De La Rosa M, Guardado ME. Improving cervical cancer prevention in a developing country. Int J Qual Health Care. 2006;18(2):81–6.
Anderson CI, Basson MD, Ali M, Davis AT, Osmer RL, McLeod MK, et al. Comprehensive multicenter graduate surgical education initiative incorporating entrustable professional activities, continuous quality improvement cycles, and a web-based platform to enhance teaching and learning. J Am Coll Surg. 2018;227(1):64–76.
Benjamin S, Seaman M. Applying continuous quality improvement and human performance technology to primary health care in Bahrain. Health Care Superv. 1998;17(1):62–71.
Byabagambi J, Marks P, Megere H, Karamagi E, Byakika S, Opio A, et al. Improving the quality of voluntary medical male circumcision through use of the continuous quality improvement approach: a pilot in 30 PEPFAR-Supported sites in Uganda. PLoS ONE. 2015;10(7):e0133369.
Hogg S, Roe Y, Mills R. Implementing evidence-based continuous quality improvement strategies in an urban Aboriginal Community Controlled Health Service in South East Queensland: a best practice implementation pilot. JBI Database Syst Rev Implement Rep. 2017;15(1):178–87.
Hopper MB, Morgan S. Continuous quality improvement initiative for pressure ulcer prevention. J Wound Ostomy Cont Nurs. 2014;41(2):178–80.
Ji J, Jiang DD, Xu Z, Yang YQ, Qian KY, Zhang MX. Continuous quality improvement of nutrition management during radiotherapy in patients with nasopharyngeal carcinoma. Nurs Open. 2021;8(6):3261–70.
Chen M, Deng JH, Zhou FD, Wang M, Wang HY. Improving the management of anemia in hemodialysis patients by implementing the continuous quality improvement program. Blood Purif. 2006;24(3):282–6.
Reeves S, Matney K, Crane V. Continuous quality improvement as an ideal in hospital practice. Health Care Superv. 1995;13(4):1–12.
Barton AJ, Danek G, Johns P, Coons M. Improving patient outcomes through CQI: vascular access planning. J Nurs Care Qual. 1998;13(2):77–85.
Buttigieg SC, Gauci D, Dey P. Continuous quality improvement in a Maltese hospital using logical framework analysis. J Health Organ Manag. 2016;30(7):1026–46.
Take N, Byakika S, Tasei H, Yoshikawa T. The effect of 5S-continuous quality improvement-total quality management approach on staff motivation, patients’ waiting time and patient satisfaction with services at hospitals in Uganda. J Public Health Afr. 2015;6(1):486.
PubMed PubMed Central Google Scholar
Jacobson GH, McCoin NS, Lescallette R, Russ S, Slovis CM. Kaizen: a method of process improvement in the emergency department. Acad Emerg Med. 2009;16(12):1341–9.
Agarwal S, Gallo J, Parashar A, Agarwal K, Ellis S, Khot U, et al. Impact of lean six sigma process improvement methodology on cardiac catheterization laboratory efficiency. Catheter Cardiovasc Interv. 2015;85:S119.
Rahul G, Samanta AK, Varaprasad G A Lean Six Sigma approach to reduce overcrowding of patients and improving the discharge process in a super-specialty hospital. In 2020 International Conference on System, Computation, Automation and Networking (ICSCAN) 2020 July 3 (pp. 1-6). IEEE
Patel J, Nattabi B, Long R, Durey A, Naoum S, Kruger E, et al. The 5 C model: A proposed continuous quality improvement framework for volunteer dental services in remote Australian Aboriginal communities. Community Dent Oral Epidemiol. 2023;51(6):1150–8.
Van Acker B, McIntosh G, Gudes M. Continuous quality improvement techniques enhance HMO members’ immunization rates. J Healthc Qual. 1998;20(2):36–41.
Horine PD, Pohjala ED, Luecke RW. Healthcare financial managers and CQI. Healthc Financ Manage. 1993;47(9):34.
Reynolds JL. Reducing the frequency of episiotomies through a continuous quality improvement program. CMAJ. 1995;153(3):275–82.
Bunik M, Galloway K, Maughlin M, Hyman D. First five quality improvement program increases adherence and continuity with well-child care. Pediatr Qual Saf. 2021;6(6):e484.
Boyle TA, MacKinnon NJ, Mahaffey T, Duggan K, Dow N. Challenges of standardized continuous quality improvement programs in community pharmacies: the case of SafetyNET-Rx. Res Social Adm Pharm. 2012;8(6):499–508.
Price A, Schwartz R, Cohen J, Manson H, Scott F. Assessing continuous quality improvement in public health: adapting lessons from healthcare. Healthc Policy. 2017;12(3):34–49.
Gage AD, Gotsadze T, Seid E, Mutasa R, Friedman J. The influence of continuous quality improvement on healthcare quality: a mixed-methods study from Zimbabwe. Soc Sci Med. 2022;298:114831.
Chan YC, Ho SJ. Continuous quality improvement: a survey of American and Canadian healthcare executives. Hosp Health Serv Adm. 1997;42(4):525–44.
Balas EA, Puryear J, Mitchell JA, Barter B. How to structure clinical practice guidelines for continuous quality improvement? J Med Syst. 1994;18(5):289–97.
ElChamaa R, Seely AJE, Jeong D, Kitto S. Barriers and facilitators to the implementation and adoption of a continuous quality improvement program in surgery: a case study. J Contin Educ Health Prof. 2022;42(4):227–35.
Candas B, Jobin G, Dubé C, Tousignant M, Abdeljelil A, Grenier S, et al. Barriers and facilitators to implementing continuous quality improvement programs in colonoscopy services: a mixed methods systematic review. Endoscopy Int Open. 2016;4(2):E118–133.
Brandrud AS, Schreiner A, Hjortdahl P, Helljesen GS, Nyen B, Nelson EC. Three success factors for continual improvement in healthcare: an analysis of the reports of improvement team members. BMJ Qual Saf. 2011;20(3):251–9.
Lee S, Choi KS, Kang HY, Cho W, Chae YM. Assessing the factors influencing continuous quality improvement implementation: experience in Korean hospitals. Int J Qual Health Care. 2002;14(5):383–91.
Horwood C, Butler L, Barker P, Phakathi S, Haskins L, Grant M, et al. A continuous quality improvement intervention to improve the effectiveness of community health workers providing care to mothers and children: a cluster randomised controlled trial in South Africa. Hum Resour Health. 2017;15(1):39.
Hyrkäs K, Lehti K. Continuous quality improvement through team supervision supported by continuous self-monitoring of work and systematic patient feedback. J Nurs Manag. 2003;11(3):177–88.
Akdemir N, Peterson LN, Campbell CM, Scheele F. Evaluation of continuous quality improvement in accreditation for medical education. BMC Med Educ. 2020;20(Suppl 1):308.
Barzansky B, Hunt D, Moineau G, Ahn D, Lai CW, Humphrey H, et al. Continuous quality improvement in an accreditation system for undergraduate medical education: benefits and challenges. Med Teach. 2015;37(11):1032–8.
Gaylis F, Nasseri R, Salmasi A, Anderson C, Mohedin S, Prime R, et al. Implementing continuous quality improvement in an integrated community urology practice: lessons learned. Urology. 2021;153:139–46.
Gaga S, Mqoqi N, Chimatira R, Moko S, Igumbor JO. Continuous quality improvement in HIV and TB services at selected healthcare facilities in South Africa. South Afr J HIV Med. 2021;22(1):1202.
Wang F, Yao D. Application effect of continuous quality improvement measures on patient satisfaction and quality of life in gynecological nursing. Am J Transl Res. 2021;13(6):6391–8.
Lee SB, Lee LL, Yeung RS, Chan J. A continuous quality improvement project to reduce medication error in the emergency department. World J Emerg Med. 2013;4(3):179–82.
Chiang AA, Lee KC, Lee JC, Wei CH. Effectiveness of a continuous quality improvement program aiming to reduce unplanned extubation: a prospective study. Intensive Care Med. 1996;22(11):1269–71.
Chinnaiyan K, Al-Mallah M, Goraya T, Patel S, Kazerooni E, Poopat C, et al. Impact of a continuous quality improvement initiative on appropriate use of coronary CT angiography: results from a multicenter, statewide registry, the advanced cardiovascular imaging consortium (ACIC). J Cardiovasc Comput Tomogr. 2011;5(4):S29–30.
Gibson-Helm M, Rumbold A, Teede H, Ranasinha S, Bailie R, Boyle J. A continuous quality improvement initiative: improving the provision of pregnancy care for Aboriginal and Torres Strait Islander women. BJOG: Int J Obstet Gynecol. 2015;122:400–1.
Bennett IM, Coco A, Anderson J, Horst M, Gambler AS, Barr WB, et al. Improving maternal care with a continuous quality improvement strategy: a report from the interventions to minimize preterm and low birth weight infants through continuous improvement techniques (IMPLICIT) network. J Am Board Fam Med. 2009;22(4):380–6.
Krall SP, Iv CLR, Donahue L. Effect of continuous quality improvement methods on reducing triage to thrombolytic interval for Acute myocardial infarction. Acad Emerg Med. 1995;2(7):603–9.
Swanson TK, Eilers GM. Physician and staff acceptance of continuous quality improvement. Fam Med. 1994;26(9):583–6.
Yu Y, Zhou Y, Wang H, Zhou T, Li Q, Li T, et al. Impact of continuous quality improvement initiatives on clinical outcomes in peritoneal dialysis. Perit Dial Int. 2014;34(Suppl 2):S43–48.
Schiff GD, Goldfield NI. Deming meets Braverman: toward a progressive analysis of the continuous quality improvement paradigm. Int J Health Serv. 1994;24(4):655–73.
American Hospital Association Division of Quality Resources Chicago, IL: The role of hospital leadership in the continuous improvement of patient care quality. American Hospital Association. J Healthc Qual. 1992;14(5):8–14,22.
Scriven M. The Logic and Methodology of checklists [dissertation]. Western Michigan University; 2000.
Hales B, Terblanche M, Fowler R, Sibbald W. Development of medical checklists for improved quality of patient care. Int J Qual Health Care. 2008;20(1):22–30.
Vermeir P, Vandijck D, Degroote S, Peleman R, Verhaeghe R, Mortier E, et al. Communication in healthcare: a narrative review of the literature and practical recommendations. Int J Clin Pract. 2015;69(11):1257–67.
Eljiz K, Greenfield D, Hogden A, Taylor R, Siddiqui N, Agaliotis M, et al. Improving knowledge translation for increased engagement and impact in healthcare. BMJ open Qual. 2020;9(3):e000983.
O’Brien JL, Shortell SM, Hughes EF, Foster RW, Carman JM, Boerstler H, et al. An integrative model for organization-wide quality improvement: lessons from the field. Qual Manage Healthc. 1995;3(4):19–30.
Adily A, Girgis S, D’Este C, Matthews V, Ward JE. Syphilis testing performance in Aboriginal primary health care: exploring impact of continuous quality improvement over time. Aust J Prim Health. 2020;26(2):178–83.
Horwood C, Butler L, Barker P, Phakathi S, Haskins L, Grant M, et al. A continuous quality improvement intervention to improve the effectiveness of community health workers providing care to mothers and children: a cluster randomised controlled trial in South Africa. Hum Resour Health. 2017;15:1–11.
Veillard J, Cowling K, Bitton A, Ratcliffe H, Kimball M, Barkley S, et al. Better measurement for performance improvement in low- and middle-income countries: the primary Health Care Performance Initiative (PHCPI) experience of conceptual framework development and indicator selection. Milbank Q. 2017;95(4):836–83.
Barbazza E, Kringos D, Kruse I, Klazinga NS, Tello JE. Creating performance intelligence for primary health care strengthening in Europe. BMC Health Serv Res. 2019;19(1):1006.
Assefa Y, Hill PS, Gilks CF, Admassu M, Tesfaye D, Van Damme W. Primary health care contributions to universal health coverage. Ethiopia Bull World Health Organ. 2020;98(12):894.
Van Weel C, Kidd MR. Why strengthening primary health care is essential to achieving universal health coverage. CMAJ. 2018;190(15):E463–466.
Download references
Acknowledgements
Not applicable.
The authors received no fund.
Author information
Authors and affiliations.
School of Public Health, The University of Queensland, Brisbane, Australia
Aklilu Endalamaw, Resham B Khatri, Tesfaye Setegn Mengistu, Daniel Erku & Yibeltal Assefa
College of Medicine and Health Sciences, Bahir Dar University, Bahir Dar, Ethiopia
Aklilu Endalamaw & Tesfaye Setegn Mengistu
Health Social Science and Development Research Institute, Kathmandu, Nepal
Resham B Khatri
Centre for Applied Health Economics, School of Medicine, Grifth University, Brisbane, Australia
Daniel Erku
Menzies Health Institute Queensland, Grifth University, Brisbane, Australia
International Institute for Primary Health Care in Ethiopia, Addis Ababa, Ethiopia
Eskinder Wolka & Anteneh Zewdie
You can also search for this author in PubMed Google Scholar
Contributions
AE conceptualized the study, developed the first draft of the manuscript, and managing feedbacks from co-authors. YA conceptualized the study, provided feedback, and supervised the whole processes. RBK provided feedback throughout. TSM provided feedback throughout. DE provided feedback throughout. EW provided feedback throughout. AZ provided feedback throughout. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Aklilu Endalamaw .
Ethics declarations
Ethics approval and consent to participate.
Not applicable because this research is based on publicly available articles.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., supplementary material 2., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Endalamaw, A., Khatri, R.B., Mengistu, T.S. et al. A scoping review of continuous quality improvement in healthcare system: conceptualization, models and tools, barriers and facilitators, and impact. BMC Health Serv Res 24 , 487 (2024). https://doi.org/10.1186/s12913-024-10828-0
Download citation
Received : 27 December 2023
Accepted : 05 March 2024
Published : 19 April 2024
DOI : https://doi.org/10.1186/s12913-024-10828-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Continuous quality improvement
- Quality of Care
BMC Health Services Research
ISSN: 1472-6963
- General enquiries: [email protected]
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Total quality management in the health-care context: integrating the literature and directing future research
Majdi m alzoubi, zm al-hamdan.
- Author information
- Article notes
- Copyright and License information
Correspondence: KS HayatiDepartment of Community Health, Faculty of Medicine and Health Sciences, University Putra Malaysia, UPM Serdang, Selangor Darul Ehsan, 43400, MalaysiaEmail [email protected]
Received 2018 Dec 4; Accepted 2019 Jul 11; Collection date 2019.
This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution – Non Commercial (unported, v3.0) License ( http://creativecommons.org/licenses/by-nc/3.0/ ). By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms ( https://www.dovepress.com/terms.php ).
Synergistic integration of predictors and elements that determine the success of total quality management (TQM) implementations in hospitals has been the bane of theoretical development in the TQM research area. Thus, this paper aims to offer a systematic literature review to provide a foundation on which research on TQM can be built and to identify the predictors of successful TQM in the health-care context.
Materials and methods
A systematic literature survey was adopted in this paper, involving the review of 25 relevant researched articles found in the databases Science Direct, EBSCO, MEDLINE, CINAHL and PubMed.
The systematic literature survey reveals five variables to be core predictors of TQM, signifying how important these variables are in the successful implementation of TQM in the health-care context. Also, it is revealed that the identified core predictors have positive effects on an improved health-care system. However, the systematic survey of the literature reveals a dearth of studies on TQM in the health-care context.
As TQM has become an important management approach for advancing effectiveness in the health-care sector, this kind of research is of value to researchers and managers. Stakeholders in the health sectors should introduce and implement TQM in hospitals and clinics. Nevertheless, this study has limitations, including that the databases and search engines adopted for the literature search are not exhaustive.
Keywords: total quality management, total quality management implementation, health care, commitment, systematic literature review, critical success factors
Introduction
Given the snowballing global economic competition and other external pressures, organizations have been compelled to pursue enduring quality and quality management which will, in turn, enhance their competitive advantage. Quality as a concept has metamorphosed over the years, and it involves objective quality bordering on the characteristics and quality of goods and services that meet implicit and explicit customer demands. It also includes subjective quality which denotes the capability to produce goods and services in the best, effective and efficient manner. 1
Looking at the health-care context, quality has always been aimed at since the time of Florence Nightingale. 2 Given that quality assurance is a requisite for economic survival, 3 and that it is an ethical, legal and social rights matter, 4 the health sector has been worried about it for more than a decade .2 Quality assurance is significant as it concerns customer satisfaction and the reduction of risks connected with health care to a minimum. 5 In the present time, health care has become a developing profession with an approach to care quality via the appraisal and regulation of structure, process and care result components. 6
Given the ever-increasing competitive and dynamic environment in which hospitals operate, and the need to augment hospitals’ performance and health-care quality, researchers 2 , 7 – 9 have conducted considerable research on enhancement of health-care quality. Moreover, given that nurse performance is crucial to the overall performance of the hospital and effective health-care system, there has been a research focus on nurse performance. 7 Nurses represent a large percentage of the health workers in any hospital. Nurses would play a significant role in the implementation of any intervention programs introduced by any hospital.
Moreover, research 8 – 11 has shown that the health-care system is facing a myriad of challenges which include high care cost, swiftly increasing dependence on technology, economic pressure on health organizations, reduction in health-care quality, 8 , 10 fulfillment of patients’ needs, 9 augmented numbers of patients who are suffering from multiple illnesses, increased demand for high-quality care, increased health-care costs and cost-containment pressures (Organization for Economic Cooperation and Development [OECD] 2007). 11 Some studies have indicated that an active way of surmounting health-care challenges is through an intervention program that will border on quality management (eg, total quality management [TQM]). 12
TQM is a system implemented by the management of an organization to achieve the satisfaction of customers/patients .13 The importance of TQM as a strategy to improve organizational performance has grown in this era of globalization. 14 Numerous research has revealed the role of TQM in the enrichment of system quality and enhancement of both employee and organizational performance. TQM is known for continuous quality improvement, quality management and total quality control. 10 TQM is held to be an innovative approach to the management of organizations. In the medical sector, TQM integrates quality orientation in all processes and procedures in health-care delivery .15 It is now being widely adopted in the medical sector of many countries. The research by Vituri and Évora 2 indicates that the literature on TQM in health sectors reveals that TQM has been fully adopted in some health institutions.
The implementation of TQM, upon which the success of TQM hinges, is intricate and complex; it depends on a good combination of certain predictors (ie, critical success factors [CSF]), and its benefits are difficult to accomplish .16 Different means of integrating predictors of TQM, although inconsistent, have emerged in the literature. 17 Some predictors have been considered crucial to TQM success, 18 and thus the exceptional predictors which can be adopted by organizations, irrespective of their industry, type, size or location. 19 These predictors are regarded as the determinants of firm performance via effective implementation of TQM.
Nevertheless, synergistic integration of predictors and elements, otherwise known as CSFs and which determine the success of TQM implementation, has been the bane of theoretical development in the TQM research area. Some of these predictors have been reported, by extant studies, 20 to have a positive impact on performance.
Likewise, substantive problems exist and can hamper theoretical development in the research area. The literature lacks foundation and structure on which the research on TQM in the health-care context is based, and connections between studies on TQM in the health-care context can hardly be drawn. The current state of extant research on TQM in the health-care context indicates that there is a need for more research in the area. 21 New knowledge development regarding identification of fitting predictors for successful TQM that enhance effectiveness in the health-care sector should be developed and where further research needs to be done should be identified.
Considering the extant works on a systematic literature review on predictors of TQM, two English written studies 14 , 22 are discernible, but Hietschold et al 14 focused on CSFs of TQM in general contexts while Aquilani et al 22 focused on the identification of TQM research, implementation of TQM research and impact-of-TQM-on-performance research in general contexts. Besides these two studies, no studies have focused on the systematic literature survey of predictors/elements of TQM in the health-care context.
Therefore, undertaking a systematic literature review in this aspect of research is germane, and this paper is poised to do as such. This paper conducts a systematic literature survey to provide a foundation stone on which research on TQM in the health-care context can be built, to evaluate the current state of evidence for TQM in the health-care context, to reveal inadequacies in the literature and to point to where further research needs to be done.
This research is guided by the following research question: what are the predictors of successful TQM in the health-care context between the period of 2005 and 2016? Like the two previous studies on a systematic literature review of TQM, this paper adopts and applies the three core steps of planning, execution and reporting that constitute a systematic literature survey. 23
This research seeks to obtain the most important predictors of successful TQM in the health-care context. This includes the review of published peer-reviewed works in English-language journals, which were published between 2005 and 2016. The literature was sourced from Science Direct, EBSCO, MEDLINE (Medical Literature Analysis and Retrieval System Online), CINAHL (Cumulative Index of Nursing and Allied Health Literature) and PubMed (US National Library of Medicine).
As part of the process of systematic literature analysis in this paper, a structured search of the academic literature was conducted to find published articles that identified TQM, total quality management, implementation, CSFs, health care and nursing. The keywords used in the search are TQM, total quality management, implementation, critical success factors, health care and nursing.
As presented in Figure 1 , a search of Science Direct, MEDLINE, EBSCO, CINAHL and PubMed yielded 2133, 6341, 1867, 7 and 474 articles, respectively. Then, repeated citations, dissertations and case studies were deleted. Via reading of the title and abstract, the remaining articles were narrowed down by relevance. Only peer-reviewed academic and practice articles that focus on total quality management, implementation, CSFs health care and nursing were selected. This exercise yielded a total of 475 articles which were published between 2005 and 2016.
Consort flow chart of systematic review method.
Abbreviation: TQM, total quality management.
Furthermore, inclusion and exclusion criteria were applied to narrow down the yielded articles. The inclusion criteria involved articles which were written in English language and published between 2005 and 2016, articles that dwell on implementation and critical factors clearly, articles from any geographical location which examined TQM, TQM principles, TQM tools and methods in the context of the health-care sector, and TQM studies that used a quantitative research approach and quasi-experimental research design. The exclusion criteria involved articles which are written in non-English language and published before 2005 or after 2016, studies in which the population and sample were not health-care workers practicing inside hospitals, gray literature or works that are not published in a peer-reviewed journal, dissertations/theses, proceedings, published abstracts, studies with qualitative research methods, and commentary articles written to convey opinion or stimulate research or discussion, with no research component. By employing these inclusion and exclusion criteria, 20 articles were generated. Moreover, to guarantee all-inclusiveness and to widen the scope of the review, a forward and backward search of citations in articles was conducted. This was recognized via the database searches, and 25 articles were finally selected. Thereafter, the 25 generated articles were fully perused.
Likewise, for exhaustive research, the approach adopted in this paper also involved the identification and measurement of predictors (CSFs) of TQM. This was done by identifying the most common or important predictors in the selected 25 works that analyze the existing models and/or scales in other contexts, industries or countries. It also includes recognition of the papers that investigate the influence of TQM implementation and/or the impact of predictors of TQM on performance. Additionally, for a proper review of the selected works, adequate plotting of the development of the line of reasoning, integrating and synthesizing the studies, authors, study design, study population, variables, measures of variables and findings of each selected article were identified and noted down. Figure 1 represents the consort flow chart of the systematic review method.
Findings and discussion
Altogether, 25 researched articles were eventually reviewed. All of the selected 25 articles are based on empirical evidence, although a possible limitation of this systematic review strategy might be the exclusion of qualitative studies in the research. Based on Table 1 , five predictors were identified. These are presented in Table 2 .
Matrix of the reviewed literature
Abbreviations: HR, human resources; TQM, total quality management.
TQM predictors in the reviewed studies
The researched literature on predictors of successful TQM implementation was found to be from various countries but in the same health sector. While some predictors adopted by a few of the researched studies were identified, the most frequent and core predictors were identified and considered. As depicted in Table 2 , education and training, continuous quality improvement, patient focus/satisfaction, top management commitment and teamwork appear to be the core predictors (CSFs) in this review. This finding validates how important these variables are in the successful implementation of TQM in the health-care context.
It is noteworthy that the core predictors (ie, education and training, continuous quality improvement, patient focus/satisfaction, top management commitment and teamwork) identified in this study were among the variables found to be central and frequently used CSFs in the previous systematic-review-based studies. 14 , 21 This validates and confirms the findings of the previous studies.
Moreover, it is found that the most adopted research method in TQM in the health-care context is cross-sectional research; 56% of the reviewed researched articles 41 – 46 used a cross-sectional research design, but 32% of the studies employed a quasi-experimental research approach. This indicates that there is still a need for more research on TQM in the health-care context which will adopt a quasi-experimental research approach, because quasi-experimental research design can be very useful in recognizing general trends from the results, and reduces the difficulty and ethical worries that may be connected with the pre-selection and random assignment of test subjects. On the geographical location aspect, the result of this analysis showed that 28% of the reviewed studies were conducted in Iran while 20% of the reviewed studies were conducted in Jordan; 12% and 8% of the reviewed studies were conducted in Saudi Arabia and Pakistan, respectively. The other studies, 4% each, came from India, Namibia, Turkey, the United States, France and Mauritius.
With regards to the influence of predictors on performance in the researched studies, it is found that all of the selected articles 47 , 48 , 49 , 50 ,. 51 that examined the effects of the core predictors (continuous quality improvement, education and training, patient focus/satisfaction, top management commitment and teamwork) of TQM indicate a positive effect of TQM in the health-care sector.
More so, the findings of this review signify that predictors of TQM implementation will result in higher levels of nurse performance .51 In addition, the literature and empirical evidence have shown that TQM in an organizational process always results in better performance of the organization. TQM focuses on patient satisfaction, organization problem identification, building and promotion of open decision-making among employees. It embraces a holistic strategy that gives room for every worker to share responsibility for the quality of the work done. It makes use of analytical mechanisms, such as flow and statistical charts and checksheets, to gather information about activities in an organization. 52 In the medical sector, TQM aims at embedding orientation of quality in all processes and procedures in the delivery of health services .15
Nevertheless, this literature survey is not an exhaustive review of the literature on TQM as it solely focused on the effect of TQM. Future research should widen the scope of this paper by including studies conducted in other contexts (eg, education, manufacturing, etc) and studies that use different research methods (eg, longitudinal research method, randomized control trial method). While TQM predictors have increased in number to reach a total of 59 TQM practices, 21 TQM predictors in the context of health care are few but growing. Investigating the nature of TQM predictors and the methods used in examining them indicates that researchers may have been keen in searching for new predictors instead of trying to cluster them and identify those that are critical for successful TQM implementation. In addition, research on TQM predictors in the health-care sector is scanty, as noted previously.
Practically, given the identified core TQM predictors in this study, it is evident that hospitals’ management should consider entrenchment of continuous quality improvement, education and training, patient focus/satisfaction top management commitment and teamwork in the implementation of TQM, which will consequently enhance hospital performance. Given that TQM predictors are many and some of them have been considered core in several specific contexts, industries, dimensions, etc, it is held that stakeholders in different sectors/industries should begin to identify the most vital TQM practices that suit their situations, goals, strategies and expected performances.
Conclusion and recommendations
As TQM has become an important management approach for advancing performance, this kind of research is of value to researchers and managers. Nevertheless, this study has limitations, including that the databases and search engines adopted for the literature search are not exhaustive. Although a good number of keywords are used, there can be other likely keywords that can be included.
This work has contributed to the enrichment of the relevant literature and made theoretical and methodological contributions. It has provided a foundation on which research on TQM can be built via review of the work done between 2005 and 2016, plotting the development of the line of reasoning, and integration and synthesis of studies from TQM in the health-care context. It has also contributed by evaluating the current state of evidence regarding TQM, indicating inadequacies in the literature and pointing to where further research needs to be done. Thus, it contributes to the present body of knowledge as well as the research on TQM in the health-care context.
This work has also established that the most adopted research method in health-care-based TQM is cross-sectional research, followed by quasi-experimental research, and the researched studies were mostly conducted in Asia. The findings of the researched literature indicate a positive effect of TQM in the health-care context, indicating that TQM implementation, which contains the identified core predictors, will result in higher levels of performance. Furthermore, TQM implementation can help health-care professionals to gain more qualified behaviors with total commitment to work toward handling the patients, which in the long run will augment their performance.
The findings of the reviewed studies indicate how it would be useful for stakeholders in the health sectors to introduce and implement TQM in the hospitals and clinics, as this would enhance the performance of the health workers and consequently improve organizational performance. Given the limitations of this work, it is sufficed to suggest that future research should widen the scope of this paper by including studies conducted in other contexts and studies that use different research methods, and it should also develop a comprehensive TQM taxonomy to explain how and why TQM practices coalesce within systems that facilitate higher performance.
The authors report no conflicts of interest in this work.
- 1. Bonechi L, Carmignani G, Mirandola R. La Gestione Della Qualità Nelle Organizzazioni-dalla Conformità All’eccellenza Gestionale . Edizioni Plus srl; 2004. [ Google Scholar ]
- 2. Vituri DW, Évora YDM. Total Quality Management and hospital nursing: an integrative literature review. Rev Bras Enferm . 2015;68(5):945–952. doi: 10.1590/0034-7167.2015680525i [ DOI ] [ Google Scholar ]
- 3. Antunes M, Sfakiotakis E. Effect of high temperature stress on ethylene biosynthesis, respiration and ripening of ‘Hayward’kiwifruit. Postharvest Biol Technol . 2000;20(3):251–259. doi: 10.1016/S0925-5214(00)00136-8 [ DOI ] [ Google Scholar ]
- 4. Adami C, Ofria C, Collier TC. Evolution of biological complexity. Proc Natl Acad Sci . 2000;97(9):4463–4468. doi: 10.1073/pnas.97.9.4463 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. Padilha KG. Iatrogenic occurrences and the quality focus. Rev Lat Am Enfermagem . 2001;9(5):91–96. doi: 10.1590/s0104-11692001000500014 [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Haddad M, Évora YDM. Qualidade da assistência de enfermagem: a opinião do paciente internado em hospital universitário público. Ciênc Cuid Saúde . 2008;7:45–52. doi: 10.4025/cienccuidsaude.v7i0.6559 [ DOI ] [ Google Scholar ]
- 7. Wright TA, Bonett DG. The moderating effects of employee tenure on the relation between organizational commitment and job performance: a meta-analysis. J Appl Psychol . 2002;87(6):1183. doi: 10.1037/0021-9010.87.6.1183 [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Aiken LH, Sermeus W, Van Den Heede K, et al. Patient safety, satisfaction, and quality of hospital care: cross sectional surveys of nurses and patients in 12 countries in Europe and the United States. BMJ . 2012;344:e1717. doi: 10.1136/bmj.e1717 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. Chang C-S, Chen S-Y, Lan Y-T. Service quality, trust, and patient satisfaction in interpersonal-based medical service encounters. BMC Health Serv Res . 2013;13(1):22. doi: 10.1186/1472-6963-13-438 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. McClellan M, Rivlin A. Improving Health while Reducing Cost Growth: What is Possible . Engelberg Center for Health Care Reform at Brookings; 2014. [ Google Scholar ]
- 11. World Health Organization. The World Health Report 2000: Health Systems: Improving Performance . World Health Organization; 2000. [ Google Scholar ]
- 12. Cummings TG, Worley CG. Organization Development and Change . Cengage learning; 2014. [ Google Scholar ]
- 13. Srima S, Wannapiroon P, Nilsook PJP-S, Sciences B. Design of total quality management information system (TQMIS) for model school on best practice. 2015;174:2160–2165. [ Google Scholar ]
- 14. Hietschold N, Reinhardt R, Gurtner S. Measuring critical success factors of TQM implementation successfully–a systematic literature review. Int J Prod Res . 2014;52(21):6254–6272. doi: 10.1080/00207543.2014.918288 [ DOI ] [ Google Scholar ]
- 15. MoHSW T. Implementation Guidelines for 5S-KAIZEN-TQM Approaches in Tanzania . Dar es Salaam (Tanzania): Ministry of Health and Social Welfare; 2013. [ Google Scholar ]
- 16. Mohammad Mosadegh Rad A. The impact of organizational culture on the successful implementation of total quality management. TQM Magazine . 2006;18(6):606–625. doi: 10.1108/09544780610707101 [ DOI ] [ Google Scholar ]
- 17. Ismail Salaheldin S. Critical success factors for TQM implementation and their impact on performance of SMEs. Int J Prod Perform Manage . 2009;58(3):215–237. doi: 10.1108/17410400910938832 [ DOI ] [ Google Scholar ]
- 18. Kumar S, Ghildayal NS, Shah RN. Examining quality and efficiency of the US healthcare system. Int J Health Care Qual Assur . 2011;24(5):366–388. doi: 10.1108/09526861111139197 [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Zairi M, Alsughayir AA. The adoption of excellence models through cultural and social adaptations: an empirical study of critical success factors and a proposed model. Total Qual Manage Bus Excellence . 2011;22(6):641–654. doi: 10.1080/14783363.2011.580654 [ DOI ] [ Google Scholar ]
- 20. Sadikoglu E, Olcay H. The effects of total quality management practices on performance and the reasons of and the barriers to TQM practices in Turkey. Adv Decis Sci . 2014;2014:1–17. doi: 10.1155/2014/537605 [ DOI ] [ Google Scholar ]
- 21. Aquilani B, Silvestri C, Ruggieri A, Gatti C. A systematic literature review on total quality management critical success factors and the identification of new avenues of research. Tqm J . 2017;29(1):184–213. doi: 10.1108/TQM-01-2016-0003 [ DOI ] [ Google Scholar ]
- 22. Aquilani B, Silvestri C, Ioppolo G, Ruggieri A. The challenging transition to bio-economies: Towards a new framework integrating corporate sustainability and value co-creation. J Clean Prod . 2018;172:4001–4009. doi: 10.1016/j.jclepro.2017.03.153 [ DOI ] [ Google Scholar ]
- 23. Tranfield D, Denyer D, Smart P. Towards a methodology for developing evidence‐informed management knowledge by means of systematic review. Br J Manage . 2003;14(3):207–222. doi: 10.1111/bjom.2003.14.issue-3 [ DOI ] [ Google Scholar ]
- 24. Irfan S, Ijaz A, Kee D, Awan M. Improving operational performance of public hospital in Pakistan: A TQM based approach. World Appl Sci J . 2012;19(6):904–913. [ Google Scholar ]
- 25. Mrayyan MT, Al‐Faouri I. Predictors of career commitment and job performance of Jordanian nurses. J Nurs Manag . 2008;16(3):246–256. doi: 10.1111/j.1365-2834.2007.00797.x [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Danial Z. Effect of Total Quality Management in Determining the Educational Needs of Critical Wards Nurses . 2009. [ Google Scholar ]
- 27. Awases MH, Bezuidenhout MC, Roos JH. Factors affecting the performance of professional nurses in Namibia. Curationis . 2013;36(1):1–8. doi: 10.4102/curationis.v36i1.108 [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Al-Ahmadi H. Factors affecting performance of hospital nurses in Riyadh Region, Saudi Arabia. Int J Health Care Qual Assur . 2009;22(1):40–54. doi: 10.1108/09526860910927943 [ DOI ] [ PubMed ] [ Google Scholar ]
- 29. Kim K, Han Y, Kwak Y, Kim J-S. Professional quality of life and clinical competencies among Korean nurses. Asian Nurs Res . 2015;9(3):200–206. doi: 10.1016/j.anr.2015.03.002 [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. AbuAlRub RF, AL‐ZARU IM. Job stress, recognition, job performance and intention to stay at work among Jordanian hospital nurses. J Nurs Manag . 2008;16(3):227–236. doi: 10.1111/j.1365-2834.2007.00810.x [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Güleryüz G, Güney S, Aydın EM, Aşan Ö. The mediating effect of job satisfaction between emotional intelligence and organisational commitment of nurses: a questionnaire survey. Int J Nurs Stud . 2008;45(11):1625–1635. doi: 10.1016/j.ijnurstu.2008.02.004 [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Lashgari MH, Arefanian S, Mohammadshahi A, Khoshdel AR. Effects of the total quality management implication on patient satisfaction in the Emergency Department of Military Hospitals. J Arch Mil Med . 2015;3(1). doi: 10.5812/jamm [ DOI ] [ Google Scholar ]
- 33. Navipour H, Nayeri ND, Hooshmand A, Zargar MT. An investigation into the effects of quality improvement method on patients’ satisfaction: a semi experimental research in Iran. Acta Med Iran . 2011;49(1):38–43. [ PubMed ] [ Google Scholar ]
- 34. Mosadeghrad AM. Developing and validating a total quality management model for healthcare organisations. TQM J . 2015;27(5):544–564. doi: 10.1108/TQM-04-2013-0051 [ DOI ] [ Google Scholar ]
- 35. Mohammad Mosadeghrad A. Why TQM does not work in Iranian healthcare organisations. Int J Health Care Qual Assur . 2014;27(4):320–335. doi: 10.1108/IJHCQA-11-2012-0110 [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Mosadeghrad AM. Implementing strategic collaborative quality management in healthcare sector. Int J Strat Change Manage . 2012;4(3/4):203–228. doi: 10.1504/IJSCM.2012.051846 [ DOI ] [ Google Scholar ]
- 37. Jones KJ, Skinner AM, High R, Reiter-Palmon R. A theory-driven, longitudinal evaluation of the impact of team training on safety culture in 24 hospitals. BMJ Qual Saf . 2013;22(5):394–404. doi: 10.1136/bmjqs-2012-000939 [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Ullah JH, Ahmed R, Malik JI, Khan MA. Outcome of 7-S, TQM technique for healthcare waste management. J Coll Physicians Surg Pak . 2011;21(12):731–734. doi: 12.2011/JCPSP.731734 [ PubMed ] [ Google Scholar ]
- 39. Padma P, Rajendran C, Sai Lokachari P. Service quality and its impact on customer satisfaction in Indian hospitals: Perspectives of patients and their attendants. Benchmarking . 2010;17(6):807–841. doi: 10.1108/14635771011089746 [ DOI ] [ Google Scholar ]
- 40. Ramseook-Munhurrun P, Munhurrun V, Panchoo A. Total Quality Management Adoption in a Public Hospital: Evidence from Mauritius . 2011. [ Google Scholar ]
- 41. Alaraki MS. The impact of critical total quality management practices on hospital performance in the ministry of health hospitals in Saudi Arabia. Qual Manage Healthcare . 2014;23(1):59–63. doi: 10.1097/QMH.0000000000000018 [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Al-Shdaifat EA. Implementation of total quality management in hospitals. J Taibah Univ Med Sci . 2015;10(4):461–466. doi: 10.1016/j.jtumed.2015.05.004 [ DOI ] [ Google Scholar ]
- 43. Larber M, Savis S. Factors affecting nurses organizational commitment. Obzornik Zdravstvene Nege . 2014;48(4):294–301. [ Google Scholar ]
- 44. Duggirala M, Rajendran C, Anantharaman R. Patient-perceived dimensions of total quality service in healthcare. Benchmarking . 2008;15(5):560–583. doi: 10.1108/14635770810903150 [ DOI ] [ Google Scholar ]
- 45. Naser Alolayyan M, Anuar Mohd Ali K, Idris F. The influence of total quality management (TQM) on operational flexibility in Jordanian hospitals: medical workers’ perspectives. Asian J Qual . 2011;12(2):204–222. doi: 10.1108/15982681111158751 [ DOI ] [ Google Scholar ]
- 46. Sweis RJ, Al-Mansour A, Tarawneh M, Al-Dweik G. The impact of total quality management practices on employee empowerment in the healthcare sector in Saudi Arabia: a study of King Khalid Hospital. Int J Prod Qual Manage . 2013;12(3):271–286. doi: 10.1504/IJPQM.2013.056149 [ DOI ] [ Google Scholar ]
- 47. Kumar R, Somrongthong R, Ahmed J. Impact of waste management training intervention on knowledge, attitude and practices of teaching hospital workers in Pakistan. Pak J Med Sci . 2016;32(3):705. doi: 10.12669/pjms.323.9903 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 48. Sagy M. Optimizing patient care processes in a children’s hospital using Six Sigma. JCOM . 2009;16:9. [ Google Scholar ]
- 49. Denker AG. Transformational Leadership in Nursing: A Pilot Nurse Leader Development Program . 2014. [ Google Scholar ]
- 50. François P, Vinck D, Labarère J, Reverdy T, Peyrin J-C. Assessment of an intervention to train teaching hospital care providers in quality management. BMJ Qual Saf . 2005;14(4):234–239. doi: 10.1136/qshc.2004.011924 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 51. El-Tohamy AE-MA, Al Raoush AT. The impact of applying total quality management principles on the overall hospital effectiveness: an empirical study on the HCAC accredited governmental hospitals in Jordan. Eur Sci J . 2015;11(10). [ Google Scholar ]
- 52. Kaluzny AD, McLaughlin CP, Simpson K. Applying total quality management concepts to public health organizations. Public Health Rep . 1992;107(3):257. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (446.8 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

Download Free PDF
QUALITY MANAGEMENT SYSTEM IN R&D: A CRITICAL LITERATURE REVIEW

Related papers
Projects create value and capabilities for organizations and beneficiaries of their outcomes and must be managed by implementing quality processes to assure an execution compliant with plans, activities, and applicable standards, and to accomplish the defined requirements and objectives in an efficient and effective manner. This study analyzed the frequency of use of quality management practices by companies with R&D units and compared them with project management students' expectations of implementing quality project management practices as a professional. The data was collected using an online survey, and twenty-six quality management practices have been analyzed using descriptive and inferential statistics, following standard procedures, and using the Independent-samples T-test. For twelve out of the twenty-six practices, significant differences have been found between the two samples, five referring to quality planning practices and seven referring to quality control practices. For the twelve quality management practices, project management students had greater expectations of implementing them, in contrast to the actual frequency of use among the surveyed companies. The results can provide inputs to improve project management practices among companies, reinforcing the importance of training and recruiting project management professionals that have the required training, talent, and aligned expectations on how to successfully manage projects.
Accreditation and Quality Assurance, 1998
Technovation, 2009
Total Quality Management & Business Excellence, 2017
Omega, 2006
Journal of Systems Architecture, 1996
Total Quality Management is the is a dynamic, multi-dimensional concept that refers not only to the mission and goals of an organization, but also works to fulfil the specific standards of the system, facility, program or event. The pedagogical the ory and practice is to determine what the quality of an organization is. In this paper the TQM in Research and Development of MNCs, Defence Organizations and Educational Institutions have been analyzed. The analysis is based on social responsibility of each sector towards the well being of the society
Problems and Perspectives in …, 2006
International Journal of …, 2006
Visione. Un altro sguardo sul mondo, 2023
Edition pro mundis, 2019
Visualization Single Guide RNA (sgRNA) of Collanic Acid Degradation Gen and Tail Fiber Protein Gen from Salmonella Phage SSE-121
Instalaciones EléctricasTema1-2, 2022
IRJET, 2023
Revista Brasileira de Estudos da Presença
International Journal of Scientific Research in Science and Technology, 2019
GROUPE SOS EDITION , 2019
Index, 2017
The Ekphrastic Review (USA), 2023
Books Journal, 2022
Ghefira Aisyah Alam, 2024
Journal of agricultural and biological science, 2012
IEEE Electron Device Letters, 2015
International Journal of Molecular Sciences, 2020
The Annals of Probability, 1980
REVELATIA: Jurnal Ilmu al-Qur`an dan Tafsir, 2021
Frontiers in education, 2024
25th IEEE VLSI Test Symmposium (VTS'07), 2007
Malaria Journal, 2020
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024

IMAGES
VIDEO
COMMENTS
QMS. ArticlePDF Available. Increasing the value of quality management systems. July 2021. International Journal of Quality and Service Sciences 13 (3) DOI: 10.1108/IJQSS-10-2020-0170. Authors: Ida ...
The paper is also an attempt to initiate research for the emerging 2030 agenda for QM, here referred to as 'Quality 2030'. ... (EMS), for example, with quality management systems (QMS), which has been a focus area with the introduction of the ISO 14000 system standards (e.g. Aboulnaga, Citation 1998). 4.5. Inspiration for research theme E ...
Quality management systems as a support for developing the quality of the offering. An improvement-oriented view of quality promotes an integrated approach for process improvement, involves the whole organisation, and has a wide range of applications, such as on service and support operations (Maguad, 2006).In a study of service employees who interact with customers, Coo and Verma (2002) found ...
Explore the latest full-text research PDFs, articles, conference papers, preprints and more on QUALITY MANAGEMENT SYSTEMS. Find methods information, sources, references or conduct a literature ...
The aim of this paper is to show the advantages of implementing a Quality Management System (QMS) in a research laboratory in order to improve the management of risks specific to research programmes and to increase the reliability of results. This paper also presents experience gained from feedback following the implementation of the Quality ...
This paper describes how quality management field has developed and evolved to date, particularly by tracking its focus over time. Design/Methodology/Approach: A systematic approach to literature ...
This paper begins with a literature review that examines the current state of TQM, the relationship among TQM, quality performance and innovation performance. ... Factual Approach to Decision Making, SA: System Approach to Management, QP: Quality Performance, IP: Innovative Performance 4.2. ... Flynn B.B., Schroeder R.G., and Sakakibara S ...
Total Quality Management (TQM) is instrumental in augmenting the quality and efficacy of healthcare service delivery, but a comprehensive evaluation of present and evolving TQM research trends within healthcare research articles is notably absent. This study provides an insightful view into the prevailing international scenarios and upcoming research frontiers in healthcare TQM research field ...
This paper presents a systematic literature review and bibliometric analyses of publications in the field of Quality Management System (QMS) by authors who applied artificial intelligence (AI) in ISO 9001:2015 audits. Scopus-indexed papers that were published from 1998 to 2021 were evaluated based on the research publication metrics made available by Scopus. From the 142 extracted Scopus ...
Survey). In organisations' quality management work, a substantial amount of time and focus is given to the quality management systems (QMS) (Elget al.,2011). Thus, it is important that QMS adds value to the organisations (Lenning and Gremyr, 2017). The interest in QMS has further grown by its potential to support sustainability
This paper is a review which presents a summary of 52 studies from 2006 to 2016 in Quality Management (QM) within Higher Education Institutes (HEIs). The aim of this paper is to submit evidence regarding the level of QM in HEIs, particularly in developing countries, and also to enhance the research in the field of QM. The findings
Total quality management (TQM) is a revolutionary approach to effective management. The research in TQM has emerged from practical needs of organizations embracing this philosophy, and the literature is mostly conceptual and practitioner‐oriented. There is a lack of sound theoretical framework classifying past efforts and guiding future research.
The ISO 9001:2015 Quality Management System is a customer-focused quality management system, so an understanding of the requirements of the ISO 9001:2015 standard will assist organizations in establishing and developing a quality management system systematically to meet customer satisfaction and continuous improvement.
Since publication in 2003 of a review 'Internal quality control: planning and implementation strategies,' 1 quality control (QC) has evolved as part of a comprehensive quality management system (QMS). The language of quality today is defined by International Standard Organization (ISO) in an effort to standardize terminology and quality management practices for world-wide applications.
Quality 4.0 is an emerging concept that has been increasingly appreciated because of the intensification of competition, continually changing customer requirements and technological evolution. It deals with aligning quality management practices with the emergent capabilities of Industry 4.0 to improve cost, time, and efficiency and increase product quality. This article aims to comprehensively ...
March 24 2013. The main purpose of article is to survey the effect of Quality Management System (QMS) on. organization efficiency based on the concept of change ma nagement. The role of top ...
The development of quality management systems (QMS) in higher education institutions (HEIs) was driven on the one hand by the competitive pressure and, on the other hand, by growing concerns from stakeholders demanding assurance of institutional program quality and their graduates. Adopting QMS is currently mandatory, and quality accreditation ...
Background The growing adoption of continuous quality improvement (CQI) initiatives in healthcare has generated a surge in research interest to gain a deeper understanding of CQI. However, comprehensive evidence regarding the diverse facets of CQI in healthcare has been limited. Our review sought to comprehensively grasp the conceptualization and principles of CQI, explore existing models and ...
TQM is a system implemented by the management of an organization to achieve the satisfaction of customers/patients.13 The importance of TQM as a strategy to improve organizational performance has grown in this era of globalization. 14 Numerous research has revealed the role of TQM in the enrichment of system quality and enhancement of both ...
effects of quality management principles and practices in the companies' quality performance. Keywords: Quality management, Performance Measures Indicators, principles, practices and Modelling. Article Classification - Research paper. V1-5 -1- ICQ'14-Tokyo, Japan 10.19-10.22, 2014
The paper concludes with a brief discussion of future work that will be necessary to develop a complete model for the application of Quality Management System to R&D. The measurement aspect of QM in the service sector is a difficult process, requiring a clear understanding of Quality and Measurement concepts.