- Chemistry Practicals
- CBSE Class 10 Chemistry Practical
- Experiments On Properties Of Acids And Bases

Experiments on Properties of Acids and Base
Table of contents.
- Reaction of Acid and Base
- Reaction of Base with a Metal
Experiment 2A – Reaction of Acid and Base
In this article, we have given step by step procedure to perform an experiment which will help you understand the different properties of acids . Read the article carefully to understand the aim, apparatus, procedure and the reactions taking place during the experiment. You can conduct the experiment and try to match the result with this.
To study the properties of acids (dilute HCl) and bases (dilute NaOH) by their reactions with the following:
- Litmus solution (red/blue)
- Solid sodium carbonate
Materials required:
- Test tube stand
- Test tube holder
- Boiling tube
- Flat bottom flask
- Thistle funnel
- Litmus paper/solution
- Fresh lime water
- Dilute NaOH
- Zinc granules
What is acid?
Chemical species which donate protons or release H + ions when dissolved in water are called acid. They turn blue litmus solution to red colour.
Hydrochloric acid reacts with zinc metal to produce zinc chloride and hydrogen gas. The reaction is given below:
Zn(s) + 2HCl(aq) → ZnCl 2 (aq) + H 2 (g)
Hydrochloric acid reacts with Na 2 CO 3 to produce carbon dioxide and turns the lime water milky as it forms calcium carbonate. The milkiness formed disappears when more than necessary carbon dioxide is passed through the solution. The reaction is as follows:
Na 2 CO 3 (s/aq) + 2HCl(aq) → 2NaCl(aq) + H 2 O(l) + CO 2 (g)
Ca(OH) 2 (aq) + CO 2 (g) → CaCO 3 (s) + H 2 O(l)
CaCO 3 (s) + H 2 O(l) + CO 2 (g) → Ca(HCO 3 ) 2 (aq)
Experimental Setup for Litmus Test Procedure:
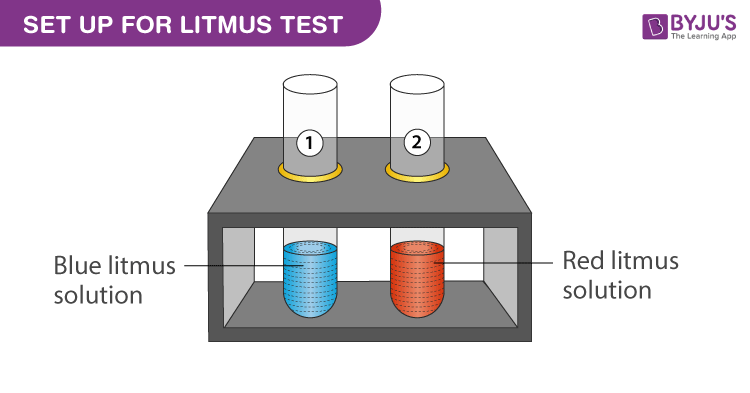
- Take a test tube stand and place two test tubes in it.
- Label the test tube as test tube 1 and test tube 2.
- Add 5 ml of blue litmus solution to test tube 1.
- Add 5 ml of red litmus solution to test tube 2.
- Use a dropper and add equal drops of hydrochloric acid in the both test tubes.
- Wait and observe the colour change.
Experimental Setup for Reaction with Zinc metal:
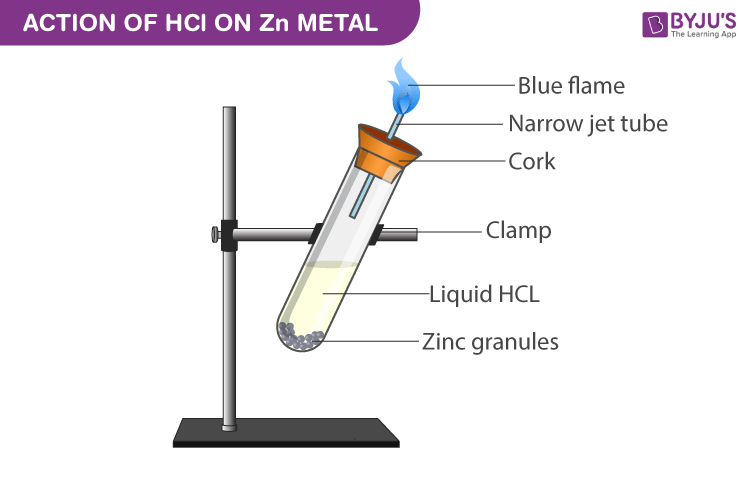
- Take a clean and dry test tube.
- Add zinc granules to it.
- Submerge the zinc granules in the test tube by adding hydrochloric acid to it.
- Close the mouth of the test tube with cork which has a glass delivery.
- A robust explosion takes place between 2-3 minutes liberating colourless and odourless gas.
- When a burning match stick is got near the glass tube mouth the gas burns with a pale blue flame with a pop sound.
2HCl(aq) + Zn(s) → ZnCl 2 (aq) + H 2 ↑
Experimental Setup for Reaction with solid sodium carbonate:
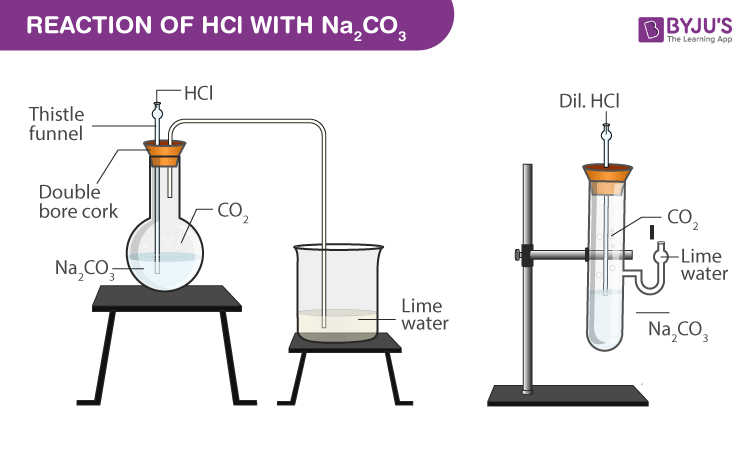
- Take a flat bottom flask with 1 gm of solid sodium carbonate and some distilled water.
- Take a clean and dry double bore cork and thistle funnel which has a delivery tube fitted to it.
- Close the mouth of the flat flask with the double bore cork.
- Add 2 mL of dilute hydrochloric acid.
- Colourless and odourless gas is liberated which is passed through the lime water using the delivery tube.
- The colour of the lime water changes to milky.
Na 2 CO 3 (s/aq) + 2HCl(aq) –→ 2NaCl(aq) + CO 2 ↑+ H 2 O(l)
Ca(OH) 2 (aq) + CO 2 ↑ –→ CaCO 3 (s) + H 2 O(l)
CaCO 3 (s) + H 2 O(l) + CO 2 (g) –→ Ca(HCO 3 ) 2 (aq)
Observation:
Result and conclusion:.
- In the litmus test experiment the blue litmus solution turns red when hydrochloric is added. Therefore, acids such as HCl show acidic character.
- Hydrochloric acid reacts with active metals such as zinc to form zinc chloride and liberate hydrogen gas.
- HCl reacts with sodium carbonate to liberate carbon dioxide gas.
Therefore, from the above three points we can conclude that HCl (Hydrochloric acid) is acidic in nature.
Precautions to be taken during the experiment:
- Conduct the experiment in clean test tubes.
- HCl is corrosive in nature and should be handled with great care.
- Take a small amount of chemicals to perform the experiments.
- While shaking the solution and reaction mixture do not spill.
- Whenever you conduct a test for hydrogen, conduct it with the least amount of gas.
- To get quick results for lime water test, pass carbon dioxide gas through the solution and shake the test tube by placing your thumb on its mouth.
Recommended Videos
Acids and bases.
Neutralization Of Acids And Bases

- When bases dissolve in water which ion do they release?Ans: OH – ions.
- Can you name a metal that reacts with base as well as acid and liberates H 2 gas?. Ans: Zinc metal.
- X releases OH – ions when it dissolves in water. Then X is?Ans: Base.
- Can you write the chemical formula for Zinc chloride?Ans: ZnCl 2 .
- What is the colour of zinc granules when it reacts with HCl?Ans: Black.
Experiment 2B – Reaction of Base with a Metal
What is base.
Chemical species which release OH – ions when dissolved in water are called bases. Sodium hydroxide is a powerful base and has a pH value greater than 7. Therefore, it turns the red litmus solution into the blue.
Sodium chloride reacts with zinc metal to produce sodium zincate and hydrogen gas. The reaction is given below:
Zn(s) + 2NaOH(aq) → Na 2 ZnO 2 (aq) + H 2 (g)
Sodium hydroxide does not react with solid sodium carbonate as both are basic in characteristic.
Na 2 CO 3 (s) + NaOH(aq) → no reaction
Sodium hydroxide neutralizes hydrochloric acid to produce salt (sodium chloride)
NaOH(aq) + HCl(aq) → NaCl(aq) + H 2 O(l)
Experimental Setup for NaOH in Litmus Test:
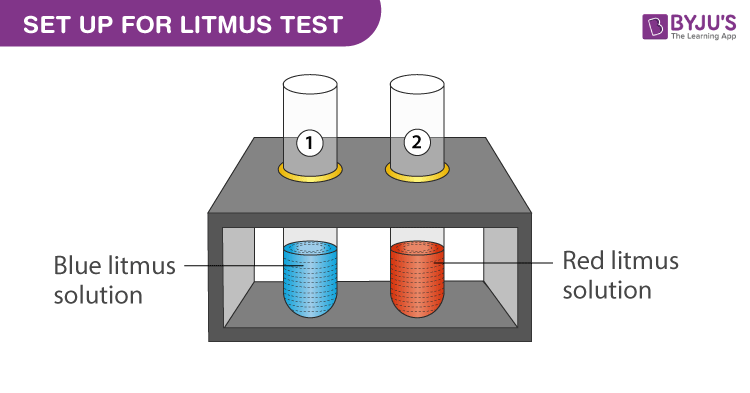
- Add 3 mL of blue litmus solution to test tube 1.
- Add 3 mL of red litmus solution to test tube 2.
- Use a dropper and add equal drops of sodium hydroxide in both the test tubes.
Experimental Setup for NaOH with Zinc metal:
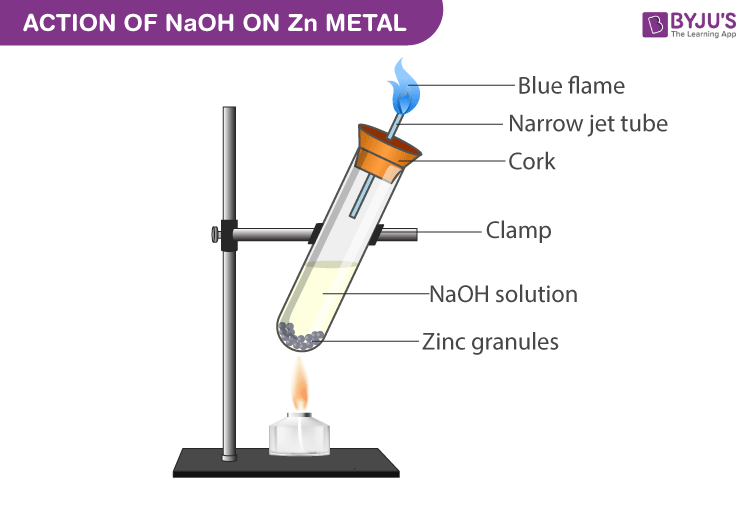
- Submerge the zinc granules in the test tube by adding sodium hydroxide to it.
- Close the test tube with cork which has a glass delivery tube.
- A robust explosion takes place between 2-3 minutes liberating odourless and colourless gas.
- When a burning match stick is in brought near the glass tube mouth the gas burns with a pale blue flame with a pop sound.
2NaOH(aq) + Zn(s) → Na 2 ZnO 2 (aq) + H 2 ↑
- Take 1 gm of solid sodium carbonate in a test tube.
- With the help of a dropper, put a few drops of NaOH in the test tube.
- No reactions are observed.
- In the litmus test experiment the red litmus solution turns into blue when sodium hydroxide is added. Therefore, bases such as NaOH show basic character.
- Sodium hydroxide reacts with active metals such as zinc to form sodium zincate and liberate hydrogen gas.
- NaOH does not react with sodium carbonate.
Therefore, from the above three points we can conclude that NaOH (sodium hydroxide) is basic in nature.
- NaOH is corrosive in nature and do not heat the mixture of zinc and NaOH to boiling point.
- Take a small amount of chemicals to perform the experiments to get the best results.
- Wash the droppers and test tubes with distilled water before and after using them in the experiment.
- Wash your hands thoroughly after the experiment.
- What happens NaOH comes in contact with red litmus solution?Ans: The red litmus solution turns into blue.
- Which metal other than aluminium reacts with NaOH to produce hydrogen gas?Ans: Zinc metal.
- When CO 2 is passed through the lime water it turns it milky. Why?Ans: The insoluble calcium carbonate makes the solution appear milky..
- Can you write the chemical formula for sodium zincate?Ans: Na 2 ZnO 2 .
- Give examples of strong bases.Ans: KOH. NaOH, etc.

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Chemistry related queries and study materials
Your result is as below
Request OTP on Voice Call

Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
it really helped me a lot to undestand the concept
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

- Andhra Pradesh
- Chhattisgarh
- West Bengal
- Madhya Pradesh
- Maharashtra
- Jammu & Kashmir
- NCERT Books 2022-23
- NCERT Solutions
- NCERT Notes
- NCERT Exemplar Books
- NCERT Exemplar Solution
- States UT Book
- School Kits & Lab Manual
- NCERT Books 2021-22
- NCERT Books 2020-21
- NCERT Book 2019-2020
- NCERT Book 2015-2016
- RD Sharma Solution
- TS Grewal Solution
- TR Jain Solution
- Selina Solution
- Frank Solution
- Lakhmir Singh and Manjit Kaur Solution
- I.E.Irodov solutions
- ICSE - Goyal Brothers Park
- ICSE - Dorothy M. Noronhe
- Micheal Vaz Solution
- S.S. Krotov Solution
- Evergreen Science
- KC Sinha Solution
- ICSE - ISC Jayanti Sengupta, Oxford
- ICSE Focus on History
- ICSE GeoGraphy Voyage
- ICSE Hindi Solution
- ICSE Treasure Trove Solution
- Thomas & Finney Solution
- SL Loney Solution
- SB Mathur Solution
- P Bahadur Solution
- Narendra Awasthi Solution
- MS Chauhan Solution
- LA Sena Solution
- Integral Calculus Amit Agarwal Solution
- IA Maron Solution
- Hall & Knight Solution
- Errorless Solution
- Pradeep's KL Gogia Solution
- OP Tandon Solutions
- Sample Papers
- Previous Year Question Paper
- Important Question
- Value Based Questions
- CBSE Syllabus
- CBSE MCQs PDF
- Assertion & Reason
- New Revision Notes
- Revision Notes
- Question Bank
- Marks Wise Question
- Toppers Answer Sheets
- Exam Paper Aalysis
- Concept Map
- CBSE Text Book
- Additional Practice Questions
- Vocational Book
- CBSE - Concept
- KVS NCERT CBSE Worksheets
- Formula Class Wise
- Formula Chapter Wise
- Toppers Notes
- Most Repeated Question
- Diagram Based Question
- Study Planner
- Competency Based Questions
- JEE Previous Year Paper
- JEE Mock Test
- JEE Crash Course
- JEE Sample Papers
- JEE Toppers Notes
- JEE Formula
- JEE Important Question
- JEE Mind Map
- JEE Integer-Numerical Type Question
- JEE Study Planner
- Important Info
- SRM-JEEE Previous Year Paper
- SRM-JEEE Mock Test
- VITEEE Previous Year Paper
- VITEEE Mock Test
- BITSAT Previous Year Paper
- BITSAT Mock Test
- Manipal Previous Year Paper
- Manipal Engineering Mock Test
- AP EAMCET Previous Year Paper
- AP EAMCET Mock Test
- COMEDK Previous Year Paper
- COMEDK Mock Test
- GUJCET Previous Year Paper
- GUJCET Mock Test
- KCET Previous Year Paper
- KCET Mock Test
- KEAM Previous Year Paper
- KEAM Mock Test
- MHT CET Previous Year Paper
- MHT CET Mock Test
- TS EAMCET Previous Year Paper
- TS EAMCET Mock Test
- WBJEE Previous Year Paper
- WBJEE Mock Test
- AMU Previous Year Paper
- AMU Mock Test
- CUSAT Previous Year Paper
- CUSAT Mock Test
- AEEE Previous Year Paper
- AEEE Mock Test
- UPSEE Previous Year Paper
- UPSEE Mock Test
- CGPET Previous Year Paper
- BCECE Previous Year Paper
- JCECE Previous Year Paper
- LPU Mock Test
- Crash Course
- Previous Year Paper
- NCERT Based Short Notes
- NCERT Based Tests
- NEET Sample Paper
- NEET Toppers Notes
- NEET Formula
- NEET Important Question
- NEET Assertion Reason Question
- NEET Study Planner
- Previous Year Papers
- Quantitative Aptitude
- Numerical Aptitude Data Interpretation
- General Knowledge
- Mathematics
- Agriculture
- Accountancy
- Business Studies
- Political science
- Enviromental Studies
- Mass Media Communication
- Teaching Aptitude
- Verbal Ability & Reading Comprehension
- Logical Reasoning & Data Interpretation
- CAT Mock Test
- CAT Important Question
- CAT Vocabulary
- CAT English Grammar
- MBA General Knowledge
- CAT Mind Map
- CAT Study Planner
- CMAT Mock Test
- SRCC GBO Mock Test
- SRCC GBO PYQs
- XAT Mock Test
- SNAP Mock Test
- IIFT Mock Test
- MAT Mock Test
- CUET PG Mock Test
- CUET PG PYQs
- MAH CET Mock Test
- MAH CET PYQs
- NAVODAYA VIDYALAYA
- SAINIK SCHOOL (AISSEE)
- Mechanical Engineering
- Electrical Engineering
- Electronics & Communication Engineering
- Civil Engineering
- Computer Science Engineering
- CBSE Board News
- Scholarship Olympiad
- School Admissions
- Entrance Exams
- All Board Updates
- Miscellaneous
- State Wise Books
- Engineering Exam
Chemistry Lab Manual Class 10 PDF Download
Free pdf download.
SHARING IS CARING If our Website helped you a little, then kindly spread our voice using Social Networks. Spread our word to your readers, friends, teachers, students & all those close ones who deserve to know what you know now.
The Chemistry Lab Manual Class 10 in PDF is a study resource that contains everything from the procedures to safety guidelines. The details given in CBSE Class 10 Chemistry Lab Manual PDF help students understand what needs to be done while in a Chemistry laboratory.
Furthermore, it helps students better prepare for the Class 10 Chemistry Practical examination and viva questions. Here on this page, we have shared the direct link to download the chapter-wise Chemistry Lab Manual Class 10 in PDF for free.
Chapter Wise NCERT Chemistry Lab Manual Class 10 PDF
Each Chapter of NCERT Class 10 Chemistry has some type of experiment or practical to perform. Therefore, the NCERT Chemistry Lab Manual Class 10 PDF is designed in a Chapter-wise manner.
It also highlights the important concepts to cover to score better marks in the Class 10 Chemistry examination as well as in the Viva test. Here are the NCERT Chemistry Lab Manual Class 10 PDF free download links in a chapter-wise manner.
- Chemistry Lab Manual Class 10 for pH Of Samples
- Chemistry Lab Manual Class 10 for Properties Of Acids & Bases
- Chemistry Lab Manual Class 10 for Types Of Reactions
- Chemistry Lab Manual Class 10 for Reactivity Series
- Chemistry Lab Manual Class 10 for Properties Of Acetic Acid
- Chemistry Lab Manual Class 10 for Soap Preparation
- Chemistry Lab Manual Class 10 for Cleaning Capacity Of Soap In Hard & Soft Water
How Class 10 Chemistry Lab Manual PDF is Designed?
Keeping in mind the importance of the subject and syllabus of Class 10 Chemistry, the Lab Manual of CBSE Class 10 Chemistry PDF is designed. There are specific details mentioned in the NCERT Class 10 Chemistry Lab Manual that all the students should know.
- Statement: The statement indicates what the practical/experiment is all about and the outcomes it is expecting.
- Apparatus/Chemicals: The PDF file of Chemistry Lab Manual Class 10 contains details of the apparatus or chemicals required to conduct the experiment.
- Theory: The related theory is explained in the Lab Manual so that students can better understand what they need to do in order to successfully conduct the practicals/experiments.
- Diagram (if applicable): To better depict the process of experiments, the Lab Manual of CBSE Class 10 Chemistry itself contains various diagrams, charts and visual images.
- Procedure: The step-by-step procedure is also mentioned in the Class 10 Chemistry Lab Manual helps students complete the experiment with ease.
- Precautions: To help students complete the experiment safely and correctly, the Lab Manual of CBSE Class 10 Chemistry has precautions given. It indicates what should be ensured by students while doing practicals or experiments.
- Viva Questions With Answers: One of the most interesting parts of the Lab Manual CBSE Class 10 is that there are viva questions with answers. Students can refer to them to prepare for the Viva test as well as the MCQ questions that will be asked in the annual examination.
Why Chemistry Lab Manual Class 10 PDF is Important to Use?
Since NCERT Chemistry Lab Manual Class 10 PDF contains standard laboratory procedures, techniques and safety measures, as well as several formulae and other helpful information, it becomes important.
The answer to the question of why Chemistry Lab Manual Class 10 PDF is important to use is that it helps students in a variety of ways to successfully complete the experiments and score impressive marks in the examinations.
How to Use NCERT Class 10 Chemistry Lab Manual PDF?
The NCERT Class 10 Chemistry Lab Manual PDF is prepared for the Class 10 students so that they can better be prepared for the practical examination therefore, students shouldn’t have such questions because the Lab Manual is already a guide. However, here is the step-by-step process of what to do with the NCERT Class 10 Chemistry Lab Manual.
- Go Through the Statement and Try to Understand: Prior to starting experimenting, students should go through the statement and understand them.
- Then Follow the Procedure as Mentioned: Followed by the statement there are theories and procedures mentioned that help students do the experiment without making any mistakes.
- Write Down the Outputs/Results: The output of Chemistry experiments should be written in the notebook. The same is mentioned in the PDF file of Chemistry Lab Manual Class 10.
- Use NCERT Class 10 Chemistry Lab Manual PDF to Prepare for the Vivas Questions: One of the best uses of the Class 10 Chemistry Lab Manual is to prepare for the Chemistry Viva questions. There are several objective types of questions in the PDF file, students should use them to revise the questions and to become ready for the CBSE Class 10 Chemistry Viva Test.
When to Use the Lab Manual of Class 10 Chemistry?
The best time to use the Lab Manual of Class 10 Chemistry is during these times:
- While Preparing the Class 10 Chemistry Practical Notebook to Submit: As per the CBSE Class 10 Chemistry course structure, all students studying Chemistry need to submit the practical notebook of Class 10 Chemistry prior to the annual examination. Since all the practicals and experiments are mentioned in the Lab Manual Class 10 Chemistry, it is the best time to use the NCERT Class 10 Chemistry Lab Manual PDF.
- During CBSE Class 10 Chemistry Viva Exam Preparation: In order to prepare for the CBSE Class 10 Chemistry viva questions, students can use the CBSE Class 10 Chemistry Lab Manual PDF. It is the best time to use the Chemistry Lab Manual because it contains various sets of MCQ questions of Chemistry ideal for the viva question.
How to Download Chemistry Lab Manual Class 10 in PDF for Free?
You can download Chemistry Lab Manual Class 10 PDF for free using the links that we provide here or the steps, we have mentioned below.
- Type “Selfstudys.com” on your device’s browser
- Once the website gets loads, click on the Navigation button
- It will show you various options to choose from, tap/click on CBSE
- Let it expand, and then tap or click on Lab Manual
- A new web page will appear, Choose your Class and then access the Chemistry Lab Manual Class 10 in PDF for free.
Skills to Learn From Chemistry Lab Manual Class 10 PDF
There are many skills to learn from Chemistry Lab Manual Class 10. A list of few skills are mentioned below:
- Observation Skills: Observation skills are crucial not only for the Class 10 annual examination but for day-to-day life. With the help of NCERT Class 10 Chemistry Lab Manual, students learn to recognize, analyze and recall the concepts they have learnt earlier.
- Problem-Solving Skills: The ability to identify problems, brainstorm, analyse answers, and implement them in experiments is one of the crucial tasks for students which they can learn by using the Class 10 Chemistry Lab Manual.
- Apparatus Handling: Chemistry experiments are done by using the apparatus which is a little tricky. However, using the Class 10 Lab Manual Chemistry students can easily learn to handle apparatus.
- Implementing Theoretical Learning into Real-life: One of the core ideas of Class 10 Lab Manual Chemistry is to help students understand how they can use their theoretical learning in real life. Many students stay confused and question people and themselves about where they can use the theory they learn the skills to use through NCERT Class 10 Chemistry Lab Manual.
International Hazard Symbols

There are nine International Hazard Symbols important for Class 10 Chemistry Students to understand. The nine warning symbols are important because they warn about dangerous materials, locations, or objects. Here’s the list of International Hazard Symbols you may find in your Chemistry Lab Manual:
Basic Laboratory Equipment
(Image Source: NCERT)
There are specific types of laboratory equipment without which many experiments or practicals aren’t possible to do. The NCERT Chemistry Lab Manual Class 10 contains complete details about all the basic laboratory equipment that you as a 11 standard student should know.
- Separating Funnels
- Ground Glass Joints
5 Reasons Chemistry Lab Manual Class 10 Is Actually a Good Thing
Among so many good reasons here are the 5 reasons why Chemistry Lab Manual Class 10 Chemistry is actually a good thing.
- It is an Official Study Resource: The official NCERT website and CBSE website have the PDF file of the CBSE Class 10 Chemistry Lab Manual. Meaning, it is an official study material offered and recommended by the board authorities.
- Based on the Latest CBSE Curriculum: All the experiments mentioned in the PDF file are being revised each year as per the CBSE curriculum and changes are made as applicable.
- Contains Questions and Answers: For the ease of Class 10 students, the Chemistry Lab Manual Class 10 in PDF contains MCQs form of questions that can be used for revision purposes as well as for the Viva exam preparation.
- Help Boost Basic Knowledge: The basic understanding in Class 10 Chemistry can be deepened by looking at real-life examples or the application of topics and knowledge in the day to day life. So, those who refer to the Class 10 Lab Manual of Chemistry can get help in boosting their basic knowledge of the subject.
- Increases Students’ Interest in the Subject: The Chemistry Class 10 Lab Manual in PDF is a resource that can help students become more curious to know about the subject. Also, the real-life uses of Chemistry theories enable students to become more interested in the subject.
What You Need to Know About Chemistry Lab Manual Class 10?
Certain things that you need to know about Chemistry Lab Manual Class 10 are -
- It is a Supportive Study Material: Keep in mind that, the Lab Manual of Class 10 CBSE is a supportive study material and therefore, it isn’t something to completely rely on.
- Help Students Prepare for Practical Examination or Internal Assessment: CBSE Class 10 Chemistry Lab Manual is one of the best study resources to be ready for the practical examination or to submit the project work for internal assessment.
- Experiment List: The lab manual of CBSE Class 10 Chemistry contains a list of experiments that students are expected to perform during their practical sessions. These experiments cover various topics of 11th Chemistry.
- Safety Precautions: The NCERT Class 10 Chemistry Lab Manual includes safety precautions that students must follow while performing the experiments. These include wearing safety goggles and lab coats, handling chemicals carefully, and following the instructions provided by the teacher and as mentioned in the Lab Manual of 11th Chemistry PDF.
What are Some of the Safety Precautions that Students Must Follow in a Chemistry Laboratory?
Since in the laboratory, safety is one of the main concerns, here are some of the safety precautions that students must follow.
- Wear Protective Gear: It is essential for students to wear appropriate protective gear such as lab coats, safety goggles, and gloves to protect their skin and eyes from any harmful substances during the practicals/experiments.
- Handle Chemicals Carefully: Students must handle all chemicals with care and use them as mentioned in the Class 10 Chemistry Lab Manual. Eating, drinking or smelling any types of chemicals are strictly prohibited as well as any physical contact with them is also prohibited.
- Avoid Contamination: Students must avoid contamination by using clean equipment, not mixing chemicals that are not supposed to be mixed. Keeping the work area clean in the Laboratory is also highly advised to avoid any kind of mishappening. It may not be mentioned directly in the Chemistry Lab Manual but experts advise it.
- Dispose of Waste Properly: In order to avoid external exposure to the chemicals, students must dispose of waste materials properly, by using designated waste disposal containers, to prevent any harm to the environment, human and animals.
- Follow Instructions: Students must follow the instructions provided in the Chemistry Lab Manual Class 10 PDF carefully and ask their teacher if they have any doubts or questions.

- NCERT Solutions for Class 12 Maths
- NCERT Solutions for Class 10 Maths
- CBSE Syllabus 2023-24
- Social Media Channels
- Login Customize Your Notification Preferences

- Second click on the toggle icon

Provide prime members with unlimited access to all study materials in PDF format.
Allow prime members to attempt MCQ tests multiple times to enhance their learning and understanding.
Provide prime users with access to exclusive PDF study materials that are not available to regular users.

