- For educators


Student Study Guide for Chemistry (11th Edition) Edit edition This problem has been solved: Solutions for Chapter 6 …
Open System : A system that can exchange mass and energy (usually in the form of heat) with its surroundings.
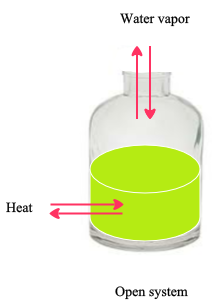
The above figure represents an open system. Both heat and water vapor are exchanging with its surroundings.
Closed System :
A system that allows the exchange of energy (usually in the form of heat) but not mass with its surroundings.
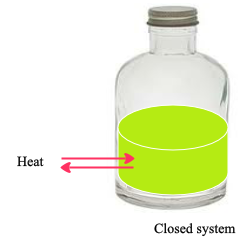
The above figure represents a closed system. We can see the exchange of heat with its surroundings.
Isolated System :
It is the system that does not allow the transfer of either mass or energy.
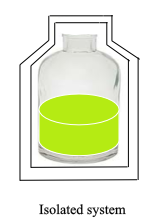
In the above figure we can see that the system is isolated from the surroundings. So, it can neither transfer mass nor energy.
Energy is the capacity to do the work. Following are the few types of energies and their definitions.
Thermal Energy: Energy associated with the random motion of atoms and molecules.
Chemical Energy : Energy stored within the structural units of chemical substances.
Potential Energy : Energy available by virtue of an object's position.
Kinetic Energy : Energy available because of the motion of an object.
Law of Conservation of Energy: The total quantity of energy in the universe is constant. All the forms of energy can be converted from one form to another form.
Corresponding textbook

- About Chegg
- Chegg For Good
- College Marketing
- Corporate Development
- Investor Relations
- Join Our Affiliate Program
- Media Center
LEGAL & POLICIES
- Advertising Choices
- Cookie Notice
- General Policies
- Intellectual Property Rights
- Terms of Use
- Global Privacy Policy
- DO NOT SELL MY INFO
- Honor Shield

CHEGG PRODUCTS AND SERVICES
- Cheap Textbooks
- Chegg Coupon
- Chegg Study Help
- College Textbooks
- Chegg Math Solver
- Mobile Apps
- Solutions Manual
- Textbook Rental
- Used Textbooks
- Digital Access Codes
- Chegg Money
CHEGG NETWORK
- Internships.com
CUSTOMER SERVICE
- Customer Service
- Give Us Feedback
- Manage Subscription
© 2003- 2024 Chegg Inc. All rights reserved.

IMAGES
VIDEO
COMMENTS
Our resource for Chemistry 11 includes answers to chapter exercises, as well as detailed information to walk you through the process step by step. With Expert Solutions for thousands of practice problems, you can take the guesswork out of studying and move forward with confidence.
Reminder: QUIZ TOMORROW Covering Topics from Part A-D (includes assignment #6) in Mole Book 1...basically, everything so far. Reminder: assignments #1 + #2 for this weekend. You should also be able to get most of your lab follow up questions done. FYI: tomorrow is photo retake day if you are needing it.
Chemistry document from Lord Byng Secondary, 8 pages, Chemistry 11 Assignment 6 Total = 40 marks Please complete the following assignment and submit to the assignment 6 folder. Acceptable file formats: word (.doc and .docx), open office, pdf, jpeg and by hand.
Chemistry 11 Assignment 6 Total = 40 marks Please complete the following assignment and submit to the assignment 6 dropbox. Acceptable file formats are: word (.doc and .docx), open office, pdf, jpeg and by hand.
Access Student Study Guide for Chemistry 11th Edition Chapter 6 solutions now. Our solutions are written by Chegg experts so you can be assured of the highest quality!
Assignment #6 - Hebden pg 52-53 Questions #33-39 +Classifying Matter Worksheet All assignments are to be completed on a separate page with the assignment number & heading.